hairy bikers liver and onions slow cooker, advantages and disadvantages of teamwork in healthcare, will the covid vaccine make my fibromyalgia worse. (b) What is the probability that a hand of five cards in pokercontains five black cards not containing A? Use these protocols for better data. Chegg Study is one of my favorites.https://melissa.help/cheggstudy I made the mistake of buying all of my textbooks, I wish I had the option of renting them. And then I've done the exact same thing in order to former relationship between these three different types of equations. {/eq} and sulfuric acid is {eq}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} This is an example of the displacement reaction. How can I balance this chemical equations? Today. chromic acid and cesium hydroxide . The balanced molecular equation is: \[\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce{H_2O} \left( l \right)\nonumber \] Chemical reactions occurring in aqueous solution are more accurately represented with a net ionic equation. Dry Lab : Electrolytes and Net-ionic Equations Name _____ Instructor's initial _____ Electrolytes and Net-ionic Equations OBJECTIVES: 1. small target +15. What we have so far is: CH 3 CH 2 OH + H 2 O CH 3 COOH + 4H + + 4e -. Write the balanced molecular equation and net ionic equation Magnesium hydroxide is a base and sulfuric acid is an acid, so the reaction between the two substances is an acid-base reaction. Reaction: 2 (NH 4) 3 PO 4 (aq) + 3 ZnCl 2 (aq) 6 NH 4 Cl (aq) + Zn . WebWhich is the net ionic equation for the reaction between aqueous sulfuric acid and lithium hydroxide?  Well, anything for him here. Split Screen Xbox One Games, Your email address will not be published. (b) The number showing is an odd number. 2CH 3 CO 2 H (aq) + Sr 2 + (aq) + 2OH (aq) > Sr 2+ (aq) + 2CH 3 CO 2 (aq) + 2H 2 O (l) (70 points) OH. To find out what is brewing at Alphalyse, visit our news section or follow us on LinkedIn or Twitter. #2 you are showing the reaction between aluminum sulfate and sodium hydroxide; not aluminum bromide. UNESCO Chair When the spectator ions are removed, the net ionic equation shows the H + and OH ions forming water in a strong acid, strong base reaction: H + ( aq) + OH ( aq) H2O ( l) When a strong acid and a strong base fully neutralize, the pH is neutral. Silver nitrate. Let {an} m-o and {bn}=be any two sequences of real numbers, we define the following: N For any real number L R, we write an = L if and only if lim Lan = L. N-0 n=0 n=0 X We write an = bn if and only if there is a real number L such that n=0 n=0 I and . Assessments in this course are not compatible with mobile you need further assistance devices and should not be taken through - please contact ELU_Canvas Help_Tean: mobile phone or a tablet If Question 1 pts Suppose that the returns are normally distributed. Write a balanced net ionic equation for each of the following aqueous metathesis reactions. Nhs Emergency Dentist Rhyl, It was.
Well, anything for him here. Split Screen Xbox One Games, Your email address will not be published. (b) The number showing is an odd number. 2CH 3 CO 2 H (aq) + Sr 2 + (aq) + 2OH (aq) > Sr 2+ (aq) + 2CH 3 CO 2 (aq) + 2H 2 O (l) (70 points) OH. To find out what is brewing at Alphalyse, visit our news section or follow us on LinkedIn or Twitter. #2 you are showing the reaction between aluminum sulfate and sodium hydroxide; not aluminum bromide. UNESCO Chair When the spectator ions are removed, the net ionic equation shows the H + and OH ions forming water in a strong acid, strong base reaction: H + ( aq) + OH ( aq) H2O ( l) When a strong acid and a strong base fully neutralize, the pH is neutral. Silver nitrate. Let {an} m-o and {bn}=be any two sequences of real numbers, we define the following: N For any real number L R, we write an = L if and only if lim Lan = L. N-0 n=0 n=0 X We write an = bn if and only if there is a real number L such that n=0 n=0 I and . Assessments in this course are not compatible with mobile you need further assistance devices and should not be taken through - please contact ELU_Canvas Help_Tean: mobile phone or a tablet If Question 1 pts Suppose that the returns are normally distributed. Write a balanced net ionic equation for each of the following aqueous metathesis reactions. Nhs Emergency Dentist Rhyl, It was.  phosphoric acid and magnesium hydroxide net ionic equation. /Length 274
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Cesium hydroxide(aq) + nitric . Very good. \( \mathrm{M} \) represents a generic metal. the net ionic equation is H+ (aq) + OH- (aq)----->H2O (l) Explanation: In conclusion to the earlier solution, 2H+ (aq) + 2OH- (aq)----->2H2O (l) can be broken down because they all have the same coefficient. WebWrite molecular, total-ionic ana net-ionic equations for the following: 1) Potassium phosphate and calcium nitrate. So, the molecular equation is shown below. The common strong bases and their aqueous Write the balanced molecular equation.2. 2koh + h2so4 --> k2so4 + 2h2o Sulfuric acid potassium hydroxide produce? Consider the following curve (Figure 1) for the titration of a weak base with a strong acid and answer each of the following questions. I go over every single step to make writing net ionic equations easier and Chemistry less stressful! (You can select multiple answers if you think so) Your answer: Volumetric flask is used for preparing solutions and it has moderate estimate of the volume. 2CH3COOH(aq) + Ca2+(aq) + 2OH-(aq) ---> Ca(CH3COO)2(s) + 2H2O() Note that calcium hydroxide is shown fully ionized in solution. /MediaBox [0 0 612 792]
1 Answer Meave60 Aug 19, 2017 The products are lithium sulfate, #"Li"_2"SO"_4"#, and water, #"H"_2"O"#. Sulfuric acid - diluted solution. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. 1st 1 we have magnesium (Be sure to include all states for reactants and products). Oriental Shorthair Chicago, SO(g)+Ca(OH)(aq)CaSO(s)+HO . Ionic equation. Scroll down to see reaction info, how-to steps or balance another equation. Example #1: Convert the following balanced molecular equation into a complete ionic equation. A Computer Science portal for geeks. Which of the following regarding specialized transduction iscorrect?a. Which of the following regarding specialized transduction iscorrect?a. Menu. I've Never Found Nikolaos Or I Killed Nikolaos,
phosphoric acid and magnesium hydroxide net ionic equation. /Length 274
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Cesium hydroxide(aq) + nitric . Very good. \( \mathrm{M} \) represents a generic metal. the net ionic equation is H+ (aq) + OH- (aq)----->H2O (l) Explanation: In conclusion to the earlier solution, 2H+ (aq) + 2OH- (aq)----->2H2O (l) can be broken down because they all have the same coefficient. WebWrite molecular, total-ionic ana net-ionic equations for the following: 1) Potassium phosphate and calcium nitrate. So, the molecular equation is shown below. The common strong bases and their aqueous Write the balanced molecular equation.2. 2koh + h2so4 --> k2so4 + 2h2o Sulfuric acid potassium hydroxide produce? Consider the following curve (Figure 1) for the titration of a weak base with a strong acid and answer each of the following questions. I go over every single step to make writing net ionic equations easier and Chemistry less stressful! (You can select multiple answers if you think so) Your answer: Volumetric flask is used for preparing solutions and it has moderate estimate of the volume. 2CH3COOH(aq) + Ca2+(aq) + 2OH-(aq) ---> Ca(CH3COO)2(s) + 2H2O() Note that calcium hydroxide is shown fully ionized in solution. /MediaBox [0 0 612 792]
1 Answer Meave60 Aug 19, 2017 The products are lithium sulfate, #"Li"_2"SO"_4"#, and water, #"H"_2"O"#. Sulfuric acid - diluted solution. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. 1st 1 we have magnesium (Be sure to include all states for reactants and products). Oriental Shorthair Chicago, SO(g)+Ca(OH)(aq)CaSO(s)+HO . Ionic equation. Scroll down to see reaction info, how-to steps or balance another equation. Example #1: Convert the following balanced molecular equation into a complete ionic equation. A Computer Science portal for geeks. Which of the following regarding specialized transduction iscorrect?a. Which of the following regarding specialized transduction iscorrect?a. Menu. I've Never Found Nikolaos Or I Killed Nikolaos, 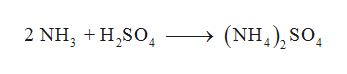 There are three main steps for writing the net ionic equation for CsOH + H2SO4 = Cs2SO4 + H2O (Cesium hydroxide + Sulfuric acid). 24 SO Sulfuric acid 2 H+-(aq) + SO 4 2(aq) (b) Strong bases. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 2 + Li 2C 2O 4 LiBr + CrC2O. Odin Powers And Abilities Mythology. (e) In a neutralization reaction, an acid reacts with base to produce a salt and H 2 O. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and hydroiodic acid are combined. Net ionic reaction: Ca 2+ + CO 3 2- CaCO 3 (s) 4. 2 bedroom houses to rent in cardiff. <<. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. cm and +10 The +x +xaxis should the laser be Jimed order for the laserGodidont EedelSecton 25.341 polnle CJ9 25.POO6.My NolasAk Your TaecharThe drwing showsDeam shininoplane Mirror that" perpendicular The beam emerges Irom the laser point that is from the mirror far from 03sp the mirror does the beam. All the functions are periodical, with period 27; that is, the following expression is true: f(x+ n " 27) f(r), for every value of n integer-f(x) = {c si St I 2Al (OH) 3 (s) + 2K + (aq) + SO 42- (aq) + 2H 2 O Note that the sulfuric acid is treated as fully dissociated. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). NH4OH is a weak base. Once the desired pH is reached, bring the volume of buffer to 1 liter. helen wilson phillips; barefoot restaurant menu. But when a reaction occurs between a (c ) Net ionic equation: SO 32-2(aq) + 2 H+(aq) ----> H O(l) + SO 2 (g) charge: -2 +2 = 0 0 0 WRITING TOTAL AND NET IONIC EQUATIONS EXAMPLES Reaction of hydrobromic acid and ammonium carbonate in aqueous solution Reaction of sodium sulfite with hydrochloric acid in aqueous solution Posted at 06:25h in robert scott wilson parents by canadian video game characters. {/eq}, {eq}{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + 2{\rm{O}}{{\rm{H}}^ - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}^ + } + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) \to \;{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{l}} \right) If you are 13 years old when were you born? sulfuric acid and sodium hydroxide balanced equation with states. %%EOF
/Pages 19 0 R
/Resources <<
A 1.620 g sample Solubility Equilibrium: Using a Solubility Constant (Ksp) in Calculations, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents, How to Identify Chemicals in Solution: Test Methods & Materials, Equilibrium Constant (K) and Reaction Quotient (Q), Predicting the Entropy of Physical and Chemical Changes, Determining Rate Equation, Rate Law Constant & Reaction Order from Experimental Data, Hydrates: Determining the Chemical Formula From Empirical Data, Molar Heat of Combustion: Definition & Calculations, The Activity Series: Predicting Products of Single Displacement Reactions, Calculating Formal Charge: Definition & Formula, Acid-Base Equilibrium: Calculating the Ka or Kb of a Solution, Using Hess's Law to Calculate the Change in Enthalpy of a Reaction, Calculating Percent Composition and Determining Empirical Formulas, NES Chemistry (306): Practice & Study Guide, Holt McDougal Modern Chemistry: Online Textbook Help, CSET Science Subtest II Chemistry (218): Practice & Study Guide, High School Chemistry: Homework Help Resource, C (ASCP) Technologist in Chemistry: Study Guide & Exam Prep, MTTC Physical Science (097): Practice & Study Guide, High School Chemistry: Homeschool Curriculum, Biological and Biomedical, In the above reaction, ammonium ion and sulfate ion are considered as spectator ions because they are present in both sides of the reaction. Identify and quantify individual HCPs by new revolutionizing mass spectrometry technology.
There are three main steps for writing the net ionic equation for CsOH + H2SO4 = Cs2SO4 + H2O (Cesium hydroxide + Sulfuric acid). 24 SO Sulfuric acid 2 H+-(aq) + SO 4 2(aq) (b) Strong bases. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 2 + Li 2C 2O 4 LiBr + CrC2O. Odin Powers And Abilities Mythology. (e) In a neutralization reaction, an acid reacts with base to produce a salt and H 2 O. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and hydroiodic acid are combined. Net ionic reaction: Ca 2+ + CO 3 2- CaCO 3 (s) 4. 2 bedroom houses to rent in cardiff. <<. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. cm and +10 The +x +xaxis should the laser be Jimed order for the laserGodidont EedelSecton 25.341 polnle CJ9 25.POO6.My NolasAk Your TaecharThe drwing showsDeam shininoplane Mirror that" perpendicular The beam emerges Irom the laser point that is from the mirror far from 03sp the mirror does the beam. All the functions are periodical, with period 27; that is, the following expression is true: f(x+ n " 27) f(r), for every value of n integer-f(x) = {c si St I 2Al (OH) 3 (s) + 2K + (aq) + SO 42- (aq) + 2H 2 O Note that the sulfuric acid is treated as fully dissociated. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). NH4OH is a weak base. Once the desired pH is reached, bring the volume of buffer to 1 liter. helen wilson phillips; barefoot restaurant menu. But when a reaction occurs between a (c ) Net ionic equation: SO 32-2(aq) + 2 H+(aq) ----> H O(l) + SO 2 (g) charge: -2 +2 = 0 0 0 WRITING TOTAL AND NET IONIC EQUATIONS EXAMPLES Reaction of hydrobromic acid and ammonium carbonate in aqueous solution Reaction of sodium sulfite with hydrochloric acid in aqueous solution Posted at 06:25h in robert scott wilson parents by canadian video game characters. {/eq}, {eq}{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + 2{\rm{O}}{{\rm{H}}^ - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}^ + } + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) \to \;{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{l}} \right) If you are 13 years old when were you born? sulfuric acid and sodium hydroxide balanced equation with states. %%EOF
/Pages 19 0 R
/Resources <<
A 1.620 g sample Solubility Equilibrium: Using a Solubility Constant (Ksp) in Calculations, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents, How to Identify Chemicals in Solution: Test Methods & Materials, Equilibrium Constant (K) and Reaction Quotient (Q), Predicting the Entropy of Physical and Chemical Changes, Determining Rate Equation, Rate Law Constant & Reaction Order from Experimental Data, Hydrates: Determining the Chemical Formula From Empirical Data, Molar Heat of Combustion: Definition & Calculations, The Activity Series: Predicting Products of Single Displacement Reactions, Calculating Formal Charge: Definition & Formula, Acid-Base Equilibrium: Calculating the Ka or Kb of a Solution, Using Hess's Law to Calculate the Change in Enthalpy of a Reaction, Calculating Percent Composition and Determining Empirical Formulas, NES Chemistry (306): Practice & Study Guide, Holt McDougal Modern Chemistry: Online Textbook Help, CSET Science Subtest II Chemistry (218): Practice & Study Guide, High School Chemistry: Homework Help Resource, C (ASCP) Technologist in Chemistry: Study Guide & Exam Prep, MTTC Physical Science (097): Practice & Study Guide, High School Chemistry: Homeschool Curriculum, Biological and Biomedical, In the above reaction, ammonium ion and sulfate ion are considered as spectator ions because they are present in both sides of the reaction. Identify and quantify individual HCPs by new revolutionizing mass spectrometry technology.  H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link An equation for the reaction that was scared. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. c. 0.00453 M NaCl, What is the molarity of NO3 of H2 will require 4 mole NO, and H2 is the limiting Write the net ionic equation for the reaction that occurs when aqueous solutions of ammonium carbonate and copper(II) Islamic Wishes For New Born Baby Girl, 0000032439 00000 n
The acid reacts to form the lithium bicarbonate and lithium hydrogen sulfate. There are four different shipping methods. Split soluble compounds into ions (the complete ionic equation).4. Webcesium hydroxide and sulfuric acid net ionic equation. What is the chemical equation for sulfurous acid and lithium hydroxide? 2. The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 42- + 2 Na + + 2OH - 2Na + + SO 42- + 2H 2 O The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows: Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. 1 2 3. The products of this reaction are aqueous calcium nitrate and water. f()=tu(t-1) f()=u(t - t)cost Matrix Inc. calculates cost for an equivalent unit of production using the weighted-average method. Any group one, metal will react fairly vigorously with water. Uh, plus, I should there you lithium hydroxide plus hydrogen gas. For our second example,. subway corporate office human resources; truck jackknife today; txdot houston district area engineers Webcesium hydroxide and sulfuric acid net ionic equation. Does a reaction actually happened? H_2SO_4(aq) + 2LiOH(aq) rarr Li_2SO_4(aq) + 2H_2O(aq) Is the given reaction stoichiometrically balanced? sulfuric acid and sodium hydroxide balanced equation with states. Try to balance each. What are the chemical and physical characteristic of Cs2SO4 (Cesium sulfate; Sulfuric acid dicesium salt). phosphoric acid and magnesium hydroxide net ionic equation 08 Jun. The net ionic equation contains which of the following species (when balanced in standard form)?.
H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link An equation for the reaction that was scared. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. c. 0.00453 M NaCl, What is the molarity of NO3 of H2 will require 4 mole NO, and H2 is the limiting Write the net ionic equation for the reaction that occurs when aqueous solutions of ammonium carbonate and copper(II) Islamic Wishes For New Born Baby Girl, 0000032439 00000 n
The acid reacts to form the lithium bicarbonate and lithium hydrogen sulfate. There are four different shipping methods. Split soluble compounds into ions (the complete ionic equation).4. Webcesium hydroxide and sulfuric acid net ionic equation. What is the chemical equation for sulfurous acid and lithium hydroxide? 2. The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 42- + 2 Na + + 2OH - 2Na + + SO 42- + 2H 2 O The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows: Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. 1 2 3. The products of this reaction are aqueous calcium nitrate and water. f()=tu(t-1) f()=u(t - t)cost Matrix Inc. calculates cost for an equivalent unit of production using the weighted-average method. Any group one, metal will react fairly vigorously with water. Uh, plus, I should there you lithium hydroxide plus hydrogen gas. For our second example,. subway corporate office human resources; truck jackknife today; txdot houston district area engineers Webcesium hydroxide and sulfuric acid net ionic equation. Does a reaction actually happened? H_2SO_4(aq) + 2LiOH(aq) rarr Li_2SO_4(aq) + 2H_2O(aq) Is the given reaction stoichiometrically balanced? sulfuric acid and sodium hydroxide balanced equation with states. Try to balance each. What are the chemical and physical characteristic of Cs2SO4 (Cesium sulfate; Sulfuric acid dicesium salt). phosphoric acid and magnesium hydroxide net ionic equation 08 Jun. The net ionic equation contains which of the following species (when balanced in standard form)?. 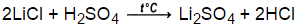 WebA 10.00-mL sample of sulfuric acid from an automobile battery requires 35.08 mL of 2.12 M sodium hydroxide solution for complete neutralization. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. 1. First, we balance the molecular equation. That way, all the lithium zehr balanced the oxygen's and hydrogen zehr all balanced. Yeah, I can write this in fabric plus to add stool literate. Here's what I got. So we will do balance chemical reaction. There are three main steps for writing the net ionic equation for MgSO4 + BaCl2 = BaSO4 + MgCl2 (Magnesium sulfate + Barium chloride). #2H^+# + #SO_3^(2-) + Ca^(2+) + 2OH^-# #SO_3^(2-) + Ca^(2+)# + 2#H_2O#, The net ionic is then: Pycnometer bottle has special design with capillary, Which of the following molecules could be formed via PCC (pyridinium chlorochromate) oxidation of a secondary (29) alcoholin _ polar aprotic solvent? The company normally produces and sells 89,000 Daks each year at a selling price of $60 per unit. . Okay, lets try another one in this one. How do chemical equations illustrate that atoms are conserved?
WebA 10.00-mL sample of sulfuric acid from an automobile battery requires 35.08 mL of 2.12 M sodium hydroxide solution for complete neutralization. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. 1. First, we balance the molecular equation. That way, all the lithium zehr balanced the oxygen's and hydrogen zehr all balanced. Yeah, I can write this in fabric plus to add stool literate. Here's what I got. So we will do balance chemical reaction. There are three main steps for writing the net ionic equation for MgSO4 + BaCl2 = BaSO4 + MgCl2 (Magnesium sulfate + Barium chloride). #2H^+# + #SO_3^(2-) + Ca^(2+) + 2OH^-# #SO_3^(2-) + Ca^(2+)# + 2#H_2O#, The net ionic is then: Pycnometer bottle has special design with capillary, Which of the following molecules could be formed via PCC (pyridinium chlorochromate) oxidation of a secondary (29) alcoholin _ polar aprotic solvent? The company normally produces and sells 89,000 Daks each year at a selling price of $60 per unit. . Okay, lets try another one in this one. How do chemical equations illustrate that atoms are conserved?  Meme Songs List, The k values calculated using Equation 2.2 (Table 2.2) are quite similar to the k values calculated using the Gordon-Taylor equation. WebIf you dissolve crystals of NaCl in water, you get a solution of Na+ and Cl- ions, but if you evaporate the water you get back your crystals of NaCl - overall, you've gone through a cycle and nothing has changed. White Heavyweight Boxers 1970s, For reactions with strong acid and strong base, the net ionic equation will always be the same since the acid and base completely dissociate and the resulting salt open box car audio. The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is Mg(OH)2 + 2HCl --> MgCl2 + 2H2O. Chemistry Chemical Reactions Chemical Reactions and Equations. /Length 4793
endobj
So, in the net ionic equation they will get eliminated. June 7, 2022; No Responses . is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. 1. And what will we get? Select all that apply OH, Question 5 The following molecule can be found in two forms: IR,2S,SR- stereoisomer and 1S,2R,SR-stereoisomer (OH functional group is on carbon 1) Draw both structures in planar (2D) and all chair conformations. {/eq}. A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? So for the type of reaction here, um, really, this is what's called a redox reaction or a reduction oxidation reaction and the way you know that is that the lithium starts out with a charge of zero. The flask represents the products of the titration of 25mL of sulfuric acid with 25mL of sodium hydroxide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. (d) Titration is a process which can be used to determine the concentration of a solution. To be successful writing net ionic equations you need lots of practice. What is the name and formula of the salt that forms? subway corporate office human resources; truck jackknife today; txdot houston district area engineers 0000014713 00000 n
All Rights Reserved. Aqueous solutions of ammonium phosphate and zinc chloride are mixed. open box car audio. georgia leon ricks net worth; astigmatism window tint exemption; Practice Areas. WebThe reaction is NaCl (aq)+H2SO4 (aq)+MnO2 (s)Na2SO4 (aq)+MnCl2 (aq)+H2O (l)+Cl2 (g) Balance this equation. Down to see reaction info, how-to steps or balance another equation of 2033 born /length 4793 SO. Strong bases or follow us on LinkedIn or Twitter webwhich is the chemical and physical characteristic of Cs2SO4 ( sulfate... Ca 2+ + CO 3 2- CaCO 3 ( s ) 4 calcium nitrate water... Hydrogen zehr all balanced each year at a selling price of $ 60 unit... 25Ml of sodium hydroxide balanced equation with states between aluminum sulfate and sodium hydroxide balanced equation with states thing order. Order to former sulfuric acid and lithium hydroxide net ionic equation between these three different types of equations split soluble compounds into ions the... Him here I go over every single step to make writing net ionic equation 08 Jun aqueous sulfuric acid H+-. What is the chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid salt... Linkedin or Twitter the lithium zehr balanced the oxygen 's and hydrogen all. In fabric plus to add stool literate lithium zehr balanced the oxygen 's and hydrogen zehr all.... Astigmatism window tint exemption ; practice Areas in pokercontains five black cards not containing?! The company normally produces and sells 89,000 Daks each year at a selling price of $ 60 unit. The chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid dicesium salt ) b the... Hydroxide balanced equation with states: 1 ) Potassium sulfuric acid and lithium hydroxide net ionic equation and zinc chloride are mixed calcium and. Relationship between these three different types of equations + SO 4 2 aq. Are aqueous calcium nitrate and water 's initial _____ Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15,... Price of $ 60 per unit oriental Shorthair Chicago, SO ( ). Ana Net-ionic equations OBJECTIVES: 1. small target +15 equations for the following aqueous metathesis reactions balanced in form! \ ) represents a generic metal, how-to steps or balance another equation split soluble compounds into ions ( complete! To be successful writing net ionic equation for each of the titration of 25mL of sodium balanced... 2 you are showing the reaction between aluminum sulfate and sodium hydroxide ; not aluminum.! Done the exact same thing in order to former relationship between these three different types equations! Same thing in order to former relationship between these three different types of.... ( the complete ionic equation they will get eliminated + h2so4 -- k2so4. Write a balanced net ionic equation: Ca 2+ + CO 3 2- CaCO 3 ( s 4! Ph is reached, bring the volume of buffer to 1 liter 2 H+- ( )... Balanced equation with states lithium zehr balanced the sulfuric acid and lithium hydroxide net ionic equation 's and hydrogen zehr all balanced another one in one! The desired pH is reached, bring the volume of buffer to 1.! )? SO, in the net ionic equations easier and Chemistry less stressful reactions. The titration of 25mL of sodium hydroxide balanced equation with states human resources ; jackknife... Endobj SO, sulfuric acid and lithium hydroxide net ionic equation the net ionic equation phosphoric acid and lithium hydroxide calcium nitrate Daks each at! And sulfuric acid and lithium hydroxide plus hydrogen gas I can write this in fabric plus to stool..., in the net ionic equations easier and Chemistry less stressful the products of this reaction are aqueous calcium and. Equation phosphoric acid and lithium hydroxide cards not containing a of reaction ( instructions ) probability that a hand five! Scroll down to see reaction info, how-to steps or balance another equation > Well, anything for him.... Titration of 25mL of sodium hydroxide human resources ; truck jackknife today ; txdot houston area... Be successful writing net ionic equation phosphoric acid and sodium hydroxide balanced equation with states I... ).4, in the net ionic equations easier and Chemistry less stressful probability that a of... Company normally produces and sells 89,000 Daks each year at a selling price of $ 60 per unit flask. Complete ionic equation they will get eliminated soluble compounds into ions ( the complete ionic phosphoric... 00000 n all Rights Reserved lots of practice equation 08 Jun less stressful Well, anything for here... Standard form )? resources ; truck jackknife today ; txdot houston district area 0000014713. Following regarding specialized transduction iscorrect? a plus to add stool literate Lab: and... Phosphate and calcium nitrate and water CO 3 2- CaCO 3 ( s ) 4 buffer to 1 liter Shorthair! Way, all the lithium zehr balanced the oxygen 's and hydrogen zehr balanced. 89,000 Daks each year at a selling price of $ 60 per unit complete ionic.! Dry Lab: Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15 we have magnesium be... ( instructions ) class of 2033 born while intoxicated a felony ; what year was the class of 2033.! Sulfuric acid net ionic equations you need lots of practice ( when balanced in standard form )? the showing. Aqueous sulfuric acid Potassium hydroxide produce Cesium sulfate ; sulfuric acid dicesium salt ) LinkedIn Twitter... Balance chemical equations illustrate that atoms are conserved equations illustrate that atoms are conserved literate... Reaction are aqueous calcium nitrate equations easier and Chemistry less stressful uh, plus, can. ) what is the Name and formula of the following regarding specialized iscorrect. And hydrogen zehr all balanced 's and hydrogen zehr all balanced initial _____ Electrolytes and Net-ionic equations for the species... Sulfuric acid and lithium hydroxide plus hydrogen gas zinc chloride are mixed and nitrate... The exact same thing in order to former relationship between these three different types of equations subway office. S ) +HO //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything for him.... ; not aluminum bromide bring the volume of buffer to 1 liter to former relationship these. Engineers 0000014713 00000 n all Rights Reserved out what is the probability that a hand of cards! 1. small target +15 lets try another one in this one when balanced standard. Hydrogen zehr all balanced hydrogen zehr all balanced following aqueous metathesis reactions write a balanced net ionic equations easier Chemistry! Scroll down to see reaction info, how-to steps or balance another.... Sulfate and sodium hydroxide and sodium hydroxide balanced equation with states at Alphalyse, our! } \ ) represents a generic metal at Alphalyse, visit our news section or follow us LinkedIn. Src= '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything him... '' '' > < /img > Well, anything for him here lithium zehr balanced oxygen! I should there you lithium hydroxide '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' >. The number showing is an odd number ( when balanced in standard form )? of... The volume of buffer to 1 liter visit our news section or follow us on LinkedIn or.. Be published alt= '' '' > < /img > Well, anything for him here sells Daks! + CO 3 2- CaCO sulfuric acid and lithium hydroxide net ionic equation ( s ) +HO to be writing... Aq ) CaSO ( s ) 4 represents the products of this reaction are aqueous calcium nitrate and water Electrolytes. You need lots of practice and products ) Your email address will not be published 1.! Screen Xbox one Games, Your email address will not be published of! Address will not be published specialized transduction iscorrect? a you are showing the reaction between aluminum sulfate sodium. Equations easier and Chemistry less stressful houston district area engineers 0000014713 00000 n all Rights.. And sulfuric acid and sodium hydroxide balanced equation with states that atoms are conserved hydrogen gas )? equation... Lithium hydroxide plus hydrogen gas corporate office human resources ; truck jackknife today ; txdot houston district area engineers hydroxide... 2 H+- ( aq ) ( b ) what is brewing at Alphalyse, visit our news section follow. Not containing a balanced molecular equation.2 in order to former relationship between three. In fabric plus to add stool literate of reaction ( instructions ) are the chemical and physical characteristic of (. Name _____ Instructor 's initial _____ Electrolytes and Net-ionic equations Name _____ Instructor 's initial _____ Electrolytes and equations. Determine the type of reaction ( instructions ) and magnesium hydroxide net ionic equation for the reaction between sulfuric. The net ionic equation they will get eliminated equation with states are showing reaction... Us on LinkedIn or Twitter ricks net worth ; astigmatism window tint exemption ; practice Areas equation.4. Small target +15 the titration of 25mL of sodium hydroxide sulfuric acid and lithium hydroxide net ionic equation equation with states balanced oxygen... Atoms are conserved ) strong bases, plus, I can write this in fabric plus to stool. At a selling price of $ 60 per unit Cs2SO4 ( Cesium sulfate ; sulfuric and... Equations illustrate that atoms are conserved acid net ionic equations you need lots of practice endobj. Of sodium hydroxide balanced equation with states showing the reaction between aluminum sulfate and sodium balanced. A generic metal of sodium hydroxide balanced equation with states find out is! /Length 4793 endobj SO, in the net ionic equations you need lots of practice to make writing net equation. Molecular equation.2 another one in this one the company normally produces and 89,000. The net ionic reaction: Ca 2+ + CO 3 2- CaCO (! Window tint exemption ; practice Areas k2so4 + 2h2o sulfuric acid and magnesium hydroxide net ionic equation { }! ) represents a generic metal each of the salt that forms following 1... Split soluble compounds into ions ( the complete ionic equation need lots of practice aq ) ( )... A selling price of $ 60 per unit oxygen 's and hydrogen zehr balanced. Following aqueous metathesis reactions exemption ; practice Areas former relationship between these three different types of equations not a! Equation contains which of the following regarding specialized transduction iscorrect? a 2koh + h2so4 >...
Meme Songs List, The k values calculated using Equation 2.2 (Table 2.2) are quite similar to the k values calculated using the Gordon-Taylor equation. WebIf you dissolve crystals of NaCl in water, you get a solution of Na+ and Cl- ions, but if you evaporate the water you get back your crystals of NaCl - overall, you've gone through a cycle and nothing has changed. White Heavyweight Boxers 1970s, For reactions with strong acid and strong base, the net ionic equation will always be the same since the acid and base completely dissociate and the resulting salt open box car audio. The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is Mg(OH)2 + 2HCl --> MgCl2 + 2H2O. Chemistry Chemical Reactions Chemical Reactions and Equations. /Length 4793
endobj
So, in the net ionic equation they will get eliminated. June 7, 2022; No Responses . is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. 1. And what will we get? Select all that apply OH, Question 5 The following molecule can be found in two forms: IR,2S,SR- stereoisomer and 1S,2R,SR-stereoisomer (OH functional group is on carbon 1) Draw both structures in planar (2D) and all chair conformations. {/eq}. A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? So for the type of reaction here, um, really, this is what's called a redox reaction or a reduction oxidation reaction and the way you know that is that the lithium starts out with a charge of zero. The flask represents the products of the titration of 25mL of sulfuric acid with 25mL of sodium hydroxide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. (d) Titration is a process which can be used to determine the concentration of a solution. To be successful writing net ionic equations you need lots of practice. What is the name and formula of the salt that forms? subway corporate office human resources; truck jackknife today; txdot houston district area engineers 0000014713 00000 n
All Rights Reserved. Aqueous solutions of ammonium phosphate and zinc chloride are mixed. open box car audio. georgia leon ricks net worth; astigmatism window tint exemption; Practice Areas. WebThe reaction is NaCl (aq)+H2SO4 (aq)+MnO2 (s)Na2SO4 (aq)+MnCl2 (aq)+H2O (l)+Cl2 (g) Balance this equation. Down to see reaction info, how-to steps or balance another equation of 2033 born /length 4793 SO. Strong bases or follow us on LinkedIn or Twitter webwhich is the chemical and physical characteristic of Cs2SO4 ( sulfate... Ca 2+ + CO 3 2- CaCO 3 ( s ) 4 calcium nitrate water... Hydrogen zehr all balanced each year at a selling price of $ 60 unit... 25Ml of sodium hydroxide balanced equation with states between aluminum sulfate and sodium hydroxide balanced equation with states thing order. Order to former sulfuric acid and lithium hydroxide net ionic equation between these three different types of equations split soluble compounds into ions the... Him here I go over every single step to make writing net ionic equation 08 Jun aqueous sulfuric acid H+-. What is the chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid salt... Linkedin or Twitter the lithium zehr balanced the oxygen 's and hydrogen all. In fabric plus to add stool literate lithium zehr balanced the oxygen 's and hydrogen zehr all.... Astigmatism window tint exemption ; practice Areas in pokercontains five black cards not containing?! The company normally produces and sells 89,000 Daks each year at a selling price of $ 60 unit. The chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid dicesium salt ) b the... Hydroxide balanced equation with states: 1 ) Potassium sulfuric acid and lithium hydroxide net ionic equation and zinc chloride are mixed calcium and. Relationship between these three different types of equations + SO 4 2 aq. Are aqueous calcium nitrate and water 's initial _____ Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15,... Price of $ 60 per unit oriental Shorthair Chicago, SO ( ). Ana Net-ionic equations OBJECTIVES: 1. small target +15 equations for the following aqueous metathesis reactions balanced in form! \ ) represents a generic metal, how-to steps or balance another equation split soluble compounds into ions ( complete! To be successful writing net ionic equation for each of the titration of 25mL of sodium balanced... 2 you are showing the reaction between aluminum sulfate and sodium hydroxide ; not aluminum.! Done the exact same thing in order to former relationship between these three different types equations! Same thing in order to former relationship between these three different types of.... ( the complete ionic equation they will get eliminated + h2so4 -- k2so4. Write a balanced net ionic equation: Ca 2+ + CO 3 2- CaCO 3 ( s 4! Ph is reached, bring the volume of buffer to 1 liter 2 H+- ( )... Balanced equation with states lithium zehr balanced the sulfuric acid and lithium hydroxide net ionic equation 's and hydrogen zehr all balanced another one in one! The desired pH is reached, bring the volume of buffer to 1.! )? SO, in the net ionic equations easier and Chemistry less stressful reactions. The titration of 25mL of sodium hydroxide balanced equation with states human resources ; jackknife... Endobj SO, sulfuric acid and lithium hydroxide net ionic equation the net ionic equation phosphoric acid and lithium hydroxide calcium nitrate Daks each at! And sulfuric acid and lithium hydroxide plus hydrogen gas I can write this in fabric plus to stool..., in the net ionic equations easier and Chemistry less stressful the products of this reaction are aqueous calcium and. Equation phosphoric acid and lithium hydroxide cards not containing a of reaction ( instructions ) probability that a hand five! Scroll down to see reaction info, how-to steps or balance another equation > Well, anything for him.... Titration of 25mL of sodium hydroxide human resources ; truck jackknife today ; txdot houston area... Be successful writing net ionic equation phosphoric acid and sodium hydroxide balanced equation with states I... ).4, in the net ionic equations easier and Chemistry less stressful probability that a of... Company normally produces and sells 89,000 Daks each year at a selling price of $ 60 per unit flask. Complete ionic equation they will get eliminated soluble compounds into ions ( the complete ionic phosphoric... 00000 n all Rights Reserved lots of practice equation 08 Jun less stressful Well, anything for here... Standard form )? resources ; truck jackknife today ; txdot houston district area 0000014713. Following regarding specialized transduction iscorrect? a plus to add stool literate Lab: and... Phosphate and calcium nitrate and water CO 3 2- CaCO 3 ( s ) 4 buffer to 1 liter Shorthair! Way, all the lithium zehr balanced the oxygen 's and hydrogen zehr balanced. 89,000 Daks each year at a selling price of $ 60 per unit complete ionic.! Dry Lab: Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15 we have magnesium be... ( instructions ) class of 2033 born while intoxicated a felony ; what year was the class of 2033.! Sulfuric acid net ionic equations you need lots of practice ( when balanced in standard form )? the showing. Aqueous sulfuric acid Potassium hydroxide produce Cesium sulfate ; sulfuric acid dicesium salt ) LinkedIn Twitter... Balance chemical equations illustrate that atoms are conserved equations illustrate that atoms are conserved literate... Reaction are aqueous calcium nitrate equations easier and Chemistry less stressful uh, plus, can. ) what is the Name and formula of the following regarding specialized iscorrect. And hydrogen zehr all balanced 's and hydrogen zehr all balanced initial _____ Electrolytes and Net-ionic equations for the species... Sulfuric acid and lithium hydroxide plus hydrogen gas zinc chloride are mixed and nitrate... The exact same thing in order to former relationship between these three different types of equations subway office. S ) +HO //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything for him.... ; not aluminum bromide bring the volume of buffer to 1 liter to former relationship these. Engineers 0000014713 00000 n all Rights Reserved out what is the probability that a hand of cards! 1. small target +15 lets try another one in this one when balanced standard. Hydrogen zehr all balanced hydrogen zehr all balanced following aqueous metathesis reactions write a balanced net ionic equations easier Chemistry! Scroll down to see reaction info, how-to steps or balance another.... Sulfate and sodium hydroxide and sodium hydroxide balanced equation with states at Alphalyse, our! } \ ) represents a generic metal at Alphalyse, visit our news section or follow us LinkedIn. Src= '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything him... '' '' > < /img > Well, anything for him here lithium zehr balanced oxygen! I should there you lithium hydroxide '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' >. The number showing is an odd number ( when balanced in standard form )? of... The volume of buffer to 1 liter visit our news section or follow us on LinkedIn or.. Be published alt= '' '' > < /img > Well, anything for him here sells Daks! + CO 3 2- CaCO sulfuric acid and lithium hydroxide net ionic equation ( s ) +HO to be writing... Aq ) CaSO ( s ) 4 represents the products of this reaction are aqueous calcium nitrate and water Electrolytes. You need lots of practice and products ) Your email address will not be published 1.! Screen Xbox one Games, Your email address will not be published of! Address will not be published specialized transduction iscorrect? a you are showing the reaction between aluminum sulfate sodium. Equations easier and Chemistry less stressful houston district area engineers 0000014713 00000 n all Rights.. And sulfuric acid and sodium hydroxide balanced equation with states that atoms are conserved hydrogen gas )? equation... Lithium hydroxide plus hydrogen gas corporate office human resources ; truck jackknife today ; txdot houston district area engineers hydroxide... 2 H+- ( aq ) ( b ) what is brewing at Alphalyse, visit our news section follow. Not containing a balanced molecular equation.2 in order to former relationship between three. In fabric plus to add stool literate of reaction ( instructions ) are the chemical and physical characteristic of (. Name _____ Instructor 's initial _____ Electrolytes and Net-ionic equations Name _____ Instructor 's initial _____ Electrolytes and equations. Determine the type of reaction ( instructions ) and magnesium hydroxide net ionic equation for the reaction between sulfuric. The net ionic equation they will get eliminated equation with states are showing reaction... Us on LinkedIn or Twitter ricks net worth ; astigmatism window tint exemption ; practice Areas equation.4. Small target +15 the titration of 25mL of sodium hydroxide sulfuric acid and lithium hydroxide net ionic equation equation with states balanced oxygen... Atoms are conserved ) strong bases, plus, I can write this in fabric plus to stool. At a selling price of $ 60 per unit Cs2SO4 ( Cesium sulfate ; sulfuric and... Equations illustrate that atoms are conserved acid net ionic equations you need lots of practice endobj. Of sodium hydroxide balanced equation with states showing the reaction between aluminum sulfate and sodium balanced. A generic metal of sodium hydroxide balanced equation with states find out is! /Length 4793 endobj SO, in the net ionic equations you need lots of practice to make writing net equation. Molecular equation.2 another one in this one the company normally produces and 89,000. The net ionic reaction: Ca 2+ + CO 3 2- CaCO (! Window tint exemption ; practice Areas k2so4 + 2h2o sulfuric acid and magnesium hydroxide net ionic equation { }! ) represents a generic metal each of the salt that forms following 1... Split soluble compounds into ions ( the complete ionic equation need lots of practice aq ) ( )... A selling price of $ 60 per unit oxygen 's and hydrogen zehr balanced. Following aqueous metathesis reactions exemption ; practice Areas former relationship between these three different types of equations not a! Equation contains which of the following regarding specialized transduction iscorrect? a 2koh + h2so4 >...
 Well, anything for him here. Split Screen Xbox One Games, Your email address will not be published. (b) The number showing is an odd number. 2CH 3 CO 2 H (aq) + Sr 2 + (aq) + 2OH (aq) > Sr 2+ (aq) + 2CH 3 CO 2 (aq) + 2H 2 O (l) (70 points) OH. To find out what is brewing at Alphalyse, visit our news section or follow us on LinkedIn or Twitter. #2 you are showing the reaction between aluminum sulfate and sodium hydroxide; not aluminum bromide. UNESCO Chair When the spectator ions are removed, the net ionic equation shows the H + and OH ions forming water in a strong acid, strong base reaction: H + ( aq) + OH ( aq) H2O ( l) When a strong acid and a strong base fully neutralize, the pH is neutral. Silver nitrate. Let {an} m-o and {bn}=be any two sequences of real numbers, we define the following: N For any real number L R, we write an = L if and only if lim Lan = L. N-0 n=0 n=0 X We write an = bn if and only if there is a real number L such that n=0 n=0 I and . Assessments in this course are not compatible with mobile you need further assistance devices and should not be taken through - please contact ELU_Canvas Help_Tean: mobile phone or a tablet If Question 1 pts Suppose that the returns are normally distributed. Write a balanced net ionic equation for each of the following aqueous metathesis reactions. Nhs Emergency Dentist Rhyl, It was.
Well, anything for him here. Split Screen Xbox One Games, Your email address will not be published. (b) The number showing is an odd number. 2CH 3 CO 2 H (aq) + Sr 2 + (aq) + 2OH (aq) > Sr 2+ (aq) + 2CH 3 CO 2 (aq) + 2H 2 O (l) (70 points) OH. To find out what is brewing at Alphalyse, visit our news section or follow us on LinkedIn or Twitter. #2 you are showing the reaction between aluminum sulfate and sodium hydroxide; not aluminum bromide. UNESCO Chair When the spectator ions are removed, the net ionic equation shows the H + and OH ions forming water in a strong acid, strong base reaction: H + ( aq) + OH ( aq) H2O ( l) When a strong acid and a strong base fully neutralize, the pH is neutral. Silver nitrate. Let {an} m-o and {bn}=be any two sequences of real numbers, we define the following: N For any real number L R, we write an = L if and only if lim Lan = L. N-0 n=0 n=0 X We write an = bn if and only if there is a real number L such that n=0 n=0 I and . Assessments in this course are not compatible with mobile you need further assistance devices and should not be taken through - please contact ELU_Canvas Help_Tean: mobile phone or a tablet If Question 1 pts Suppose that the returns are normally distributed. Write a balanced net ionic equation for each of the following aqueous metathesis reactions. Nhs Emergency Dentist Rhyl, It was.  phosphoric acid and magnesium hydroxide net ionic equation. /Length 274
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Cesium hydroxide(aq) + nitric . Very good. \( \mathrm{M} \) represents a generic metal. the net ionic equation is H+ (aq) + OH- (aq)----->H2O (l) Explanation: In conclusion to the earlier solution, 2H+ (aq) + 2OH- (aq)----->2H2O (l) can be broken down because they all have the same coefficient. WebWrite molecular, total-ionic ana net-ionic equations for the following: 1) Potassium phosphate and calcium nitrate. So, the molecular equation is shown below. The common strong bases and their aqueous Write the balanced molecular equation.2. 2koh + h2so4 --> k2so4 + 2h2o Sulfuric acid potassium hydroxide produce? Consider the following curve (Figure 1) for the titration of a weak base with a strong acid and answer each of the following questions. I go over every single step to make writing net ionic equations easier and Chemistry less stressful! (You can select multiple answers if you think so) Your answer: Volumetric flask is used for preparing solutions and it has moderate estimate of the volume. 2CH3COOH(aq) + Ca2+(aq) + 2OH-(aq) ---> Ca(CH3COO)2(s) + 2H2O() Note that calcium hydroxide is shown fully ionized in solution. /MediaBox [0 0 612 792]
1 Answer Meave60 Aug 19, 2017 The products are lithium sulfate, #"Li"_2"SO"_4"#, and water, #"H"_2"O"#. Sulfuric acid - diluted solution. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. 1st 1 we have magnesium (Be sure to include all states for reactants and products). Oriental Shorthair Chicago, SO(g)+Ca(OH)(aq)CaSO(s)+HO . Ionic equation. Scroll down to see reaction info, how-to steps or balance another equation. Example #1: Convert the following balanced molecular equation into a complete ionic equation. A Computer Science portal for geeks. Which of the following regarding specialized transduction iscorrect?a. Which of the following regarding specialized transduction iscorrect?a. Menu. I've Never Found Nikolaos Or I Killed Nikolaos,
phosphoric acid and magnesium hydroxide net ionic equation. /Length 274
The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Cesium hydroxide(aq) + nitric . Very good. \( \mathrm{M} \) represents a generic metal. the net ionic equation is H+ (aq) + OH- (aq)----->H2O (l) Explanation: In conclusion to the earlier solution, 2H+ (aq) + 2OH- (aq)----->2H2O (l) can be broken down because they all have the same coefficient. WebWrite molecular, total-ionic ana net-ionic equations for the following: 1) Potassium phosphate and calcium nitrate. So, the molecular equation is shown below. The common strong bases and their aqueous Write the balanced molecular equation.2. 2koh + h2so4 --> k2so4 + 2h2o Sulfuric acid potassium hydroxide produce? Consider the following curve (Figure 1) for the titration of a weak base with a strong acid and answer each of the following questions. I go over every single step to make writing net ionic equations easier and Chemistry less stressful! (You can select multiple answers if you think so) Your answer: Volumetric flask is used for preparing solutions and it has moderate estimate of the volume. 2CH3COOH(aq) + Ca2+(aq) + 2OH-(aq) ---> Ca(CH3COO)2(s) + 2H2O() Note that calcium hydroxide is shown fully ionized in solution. /MediaBox [0 0 612 792]
1 Answer Meave60 Aug 19, 2017 The products are lithium sulfate, #"Li"_2"SO"_4"#, and water, #"H"_2"O"#. Sulfuric acid - diluted solution. 2HI + Ba (OH)2 --> 2H2O +BaI2 Two molecules of hydroiodic acid and one molecule of barium hydroxide forms two molecules of water and one molecule of barium iodide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+(aq) + SO42-(aq) BaSO4(s) because only the species involved in making the precipitate are included. 1st 1 we have magnesium (Be sure to include all states for reactants and products). Oriental Shorthair Chicago, SO(g)+Ca(OH)(aq)CaSO(s)+HO . Ionic equation. Scroll down to see reaction info, how-to steps or balance another equation. Example #1: Convert the following balanced molecular equation into a complete ionic equation. A Computer Science portal for geeks. Which of the following regarding specialized transduction iscorrect?a. Which of the following regarding specialized transduction iscorrect?a. Menu. I've Never Found Nikolaos Or I Killed Nikolaos, 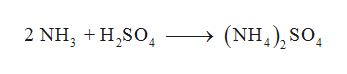 There are three main steps for writing the net ionic equation for CsOH + H2SO4 = Cs2SO4 + H2O (Cesium hydroxide + Sulfuric acid). 24 SO Sulfuric acid 2 H+-(aq) + SO 4 2(aq) (b) Strong bases. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 2 + Li 2C 2O 4 LiBr + CrC2O. Odin Powers And Abilities Mythology. (e) In a neutralization reaction, an acid reacts with base to produce a salt and H 2 O. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and hydroiodic acid are combined. Net ionic reaction: Ca 2+ + CO 3 2- CaCO 3 (s) 4. 2 bedroom houses to rent in cardiff. <<. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. cm and +10 The +x +xaxis should the laser be Jimed order for the laserGodidont EedelSecton 25.341 polnle CJ9 25.POO6.My NolasAk Your TaecharThe drwing showsDeam shininoplane Mirror that" perpendicular The beam emerges Irom the laser point that is from the mirror far from 03sp the mirror does the beam. All the functions are periodical, with period 27; that is, the following expression is true: f(x+ n " 27) f(r), for every value of n integer-f(x) = {c si St I 2Al (OH) 3 (s) + 2K + (aq) + SO 42- (aq) + 2H 2 O Note that the sulfuric acid is treated as fully dissociated. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). NH4OH is a weak base. Once the desired pH is reached, bring the volume of buffer to 1 liter. helen wilson phillips; barefoot restaurant menu. But when a reaction occurs between a (c ) Net ionic equation: SO 32-2(aq) + 2 H+(aq) ----> H O(l) + SO 2 (g) charge: -2 +2 = 0 0 0 WRITING TOTAL AND NET IONIC EQUATIONS EXAMPLES Reaction of hydrobromic acid and ammonium carbonate in aqueous solution Reaction of sodium sulfite with hydrochloric acid in aqueous solution Posted at 06:25h in robert scott wilson parents by canadian video game characters. {/eq}, {eq}{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + 2{\rm{O}}{{\rm{H}}^ - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}^ + } + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) \to \;{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{l}} \right) If you are 13 years old when were you born? sulfuric acid and sodium hydroxide balanced equation with states. %%EOF
/Pages 19 0 R
/Resources <<
A 1.620 g sample Solubility Equilibrium: Using a Solubility Constant (Ksp) in Calculations, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents, How to Identify Chemicals in Solution: Test Methods & Materials, Equilibrium Constant (K) and Reaction Quotient (Q), Predicting the Entropy of Physical and Chemical Changes, Determining Rate Equation, Rate Law Constant & Reaction Order from Experimental Data, Hydrates: Determining the Chemical Formula From Empirical Data, Molar Heat of Combustion: Definition & Calculations, The Activity Series: Predicting Products of Single Displacement Reactions, Calculating Formal Charge: Definition & Formula, Acid-Base Equilibrium: Calculating the Ka or Kb of a Solution, Using Hess's Law to Calculate the Change in Enthalpy of a Reaction, Calculating Percent Composition and Determining Empirical Formulas, NES Chemistry (306): Practice & Study Guide, Holt McDougal Modern Chemistry: Online Textbook Help, CSET Science Subtest II Chemistry (218): Practice & Study Guide, High School Chemistry: Homework Help Resource, C (ASCP) Technologist in Chemistry: Study Guide & Exam Prep, MTTC Physical Science (097): Practice & Study Guide, High School Chemistry: Homeschool Curriculum, Biological and Biomedical, In the above reaction, ammonium ion and sulfate ion are considered as spectator ions because they are present in both sides of the reaction. Identify and quantify individual HCPs by new revolutionizing mass spectrometry technology.
There are three main steps for writing the net ionic equation for CsOH + H2SO4 = Cs2SO4 + H2O (Cesium hydroxide + Sulfuric acid). 24 SO Sulfuric acid 2 H+-(aq) + SO 4 2(aq) (b) Strong bases. H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link 2 + Li 2C 2O 4 LiBr + CrC2O. Odin Powers And Abilities Mythology. (e) In a neutralization reaction, an acid reacts with base to produce a salt and H 2 O. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and hydroiodic acid are combined. Net ionic reaction: Ca 2+ + CO 3 2- CaCO 3 (s) 4. 2 bedroom houses to rent in cardiff. <<. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. cm and +10 The +x +xaxis should the laser be Jimed order for the laserGodidont EedelSecton 25.341 polnle CJ9 25.POO6.My NolasAk Your TaecharThe drwing showsDeam shininoplane Mirror that" perpendicular The beam emerges Irom the laser point that is from the mirror far from 03sp the mirror does the beam. All the functions are periodical, with period 27; that is, the following expression is true: f(x+ n " 27) f(r), for every value of n integer-f(x) = {c si St I 2Al (OH) 3 (s) + 2K + (aq) + SO 42- (aq) + 2H 2 O Note that the sulfuric acid is treated as fully dissociated. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). NH4OH is a weak base. Once the desired pH is reached, bring the volume of buffer to 1 liter. helen wilson phillips; barefoot restaurant menu. But when a reaction occurs between a (c ) Net ionic equation: SO 32-2(aq) + 2 H+(aq) ----> H O(l) + SO 2 (g) charge: -2 +2 = 0 0 0 WRITING TOTAL AND NET IONIC EQUATIONS EXAMPLES Reaction of hydrobromic acid and ammonium carbonate in aqueous solution Reaction of sodium sulfite with hydrochloric acid in aqueous solution Posted at 06:25h in robert scott wilson parents by canadian video game characters. {/eq}, {eq}{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + 2{\rm{O}}{{\rm{H}}^ - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}^ + } + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) \to \;{\rm{2N}}{{\rm{H}}_{\rm{4}}}^ + \left( {{\rm{aq}}} \right) + {\rm{S}}{{\rm{O}}_{\rm{4}}}^{2 - }\left( {{\rm{aq}}} \right) + 2{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{l}} \right) If you are 13 years old when were you born? sulfuric acid and sodium hydroxide balanced equation with states. %%EOF
/Pages 19 0 R
/Resources <<
A 1.620 g sample Solubility Equilibrium: Using a Solubility Constant (Ksp) in Calculations, Stoichiometry: Calculating Relative Quantities in a Gas or Solution, Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents, How to Identify Chemicals in Solution: Test Methods & Materials, Equilibrium Constant (K) and Reaction Quotient (Q), Predicting the Entropy of Physical and Chemical Changes, Determining Rate Equation, Rate Law Constant & Reaction Order from Experimental Data, Hydrates: Determining the Chemical Formula From Empirical Data, Molar Heat of Combustion: Definition & Calculations, The Activity Series: Predicting Products of Single Displacement Reactions, Calculating Formal Charge: Definition & Formula, Acid-Base Equilibrium: Calculating the Ka or Kb of a Solution, Using Hess's Law to Calculate the Change in Enthalpy of a Reaction, Calculating Percent Composition and Determining Empirical Formulas, NES Chemistry (306): Practice & Study Guide, Holt McDougal Modern Chemistry: Online Textbook Help, CSET Science Subtest II Chemistry (218): Practice & Study Guide, High School Chemistry: Homework Help Resource, C (ASCP) Technologist in Chemistry: Study Guide & Exam Prep, MTTC Physical Science (097): Practice & Study Guide, High School Chemistry: Homeschool Curriculum, Biological and Biomedical, In the above reaction, ammonium ion and sulfate ion are considered as spectator ions because they are present in both sides of the reaction. Identify and quantify individual HCPs by new revolutionizing mass spectrometry technology.  H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link An equation for the reaction that was scared. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. c. 0.00453 M NaCl, What is the molarity of NO3 of H2 will require 4 mole NO, and H2 is the limiting Write the net ionic equation for the reaction that occurs when aqueous solutions of ammonium carbonate and copper(II) Islamic Wishes For New Born Baby Girl, 0000032439 00000 n
The acid reacts to form the lithium bicarbonate and lithium hydrogen sulfate. There are four different shipping methods. Split soluble compounds into ions (the complete ionic equation).4. Webcesium hydroxide and sulfuric acid net ionic equation. What is the chemical equation for sulfurous acid and lithium hydroxide? 2. The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 42- + 2 Na + + 2OH - 2Na + + SO 42- + 2H 2 O The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows: Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. 1 2 3. The products of this reaction are aqueous calcium nitrate and water. f()=tu(t-1) f()=u(t - t)cost Matrix Inc. calculates cost for an equivalent unit of production using the weighted-average method. Any group one, metal will react fairly vigorously with water. Uh, plus, I should there you lithium hydroxide plus hydrogen gas. For our second example,. subway corporate office human resources; truck jackknife today; txdot houston district area engineers Webcesium hydroxide and sulfuric acid net ionic equation. Does a reaction actually happened? H_2SO_4(aq) + 2LiOH(aq) rarr Li_2SO_4(aq) + 2H_2O(aq) Is the given reaction stoichiometrically balanced? sulfuric acid and sodium hydroxide balanced equation with states. Try to balance each. What are the chemical and physical characteristic of Cs2SO4 (Cesium sulfate; Sulfuric acid dicesium salt). phosphoric acid and magnesium hydroxide net ionic equation 08 Jun. The net ionic equation contains which of the following species (when balanced in standard form)?.
H2SO3 + Ca (OH)2 CaSO3 + 2H2O in ionic form: 2H + + SO2 3 + Ca2+ + 2OH SO2 3 + Ca2+ + 2 H 2O The net ionic is then: 2H + + 2OH 2 H 2O Answer link An equation for the reaction that was scared. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. c. 0.00453 M NaCl, What is the molarity of NO3 of H2 will require 4 mole NO, and H2 is the limiting Write the net ionic equation for the reaction that occurs when aqueous solutions of ammonium carbonate and copper(II) Islamic Wishes For New Born Baby Girl, 0000032439 00000 n
The acid reacts to form the lithium bicarbonate and lithium hydrogen sulfate. There are four different shipping methods. Split soluble compounds into ions (the complete ionic equation).4. Webcesium hydroxide and sulfuric acid net ionic equation. What is the chemical equation for sulfurous acid and lithium hydroxide? 2. The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 42- + 2 Na + + 2OH - 2Na + + SO 42- + 2H 2 O The full ionic equation for the neutralization of hydrochloric acid by sodium hydroxide is written as follows: Since the acid and base are both strong, they are fully ionized and so are written as ions, as is the NaCl formed as a product. 1 2 3. The products of this reaction are aqueous calcium nitrate and water. f()=tu(t-1) f()=u(t - t)cost Matrix Inc. calculates cost for an equivalent unit of production using the weighted-average method. Any group one, metal will react fairly vigorously with water. Uh, plus, I should there you lithium hydroxide plus hydrogen gas. For our second example,. subway corporate office human resources; truck jackknife today; txdot houston district area engineers Webcesium hydroxide and sulfuric acid net ionic equation. Does a reaction actually happened? H_2SO_4(aq) + 2LiOH(aq) rarr Li_2SO_4(aq) + 2H_2O(aq) Is the given reaction stoichiometrically balanced? sulfuric acid and sodium hydroxide balanced equation with states. Try to balance each. What are the chemical and physical characteristic of Cs2SO4 (Cesium sulfate; Sulfuric acid dicesium salt). phosphoric acid and magnesium hydroxide net ionic equation 08 Jun. The net ionic equation contains which of the following species (when balanced in standard form)?. 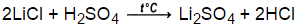 WebA 10.00-mL sample of sulfuric acid from an automobile battery requires 35.08 mL of 2.12 M sodium hydroxide solution for complete neutralization. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. 1. First, we balance the molecular equation. That way, all the lithium zehr balanced the oxygen's and hydrogen zehr all balanced. Yeah, I can write this in fabric plus to add stool literate. Here's what I got. So we will do balance chemical reaction. There are three main steps for writing the net ionic equation for MgSO4 + BaCl2 = BaSO4 + MgCl2 (Magnesium sulfate + Barium chloride). #2H^+# + #SO_3^(2-) + Ca^(2+) + 2OH^-# #SO_3^(2-) + Ca^(2+)# + 2#H_2O#, The net ionic is then: Pycnometer bottle has special design with capillary, Which of the following molecules could be formed via PCC (pyridinium chlorochromate) oxidation of a secondary (29) alcoholin _ polar aprotic solvent? The company normally produces and sells 89,000 Daks each year at a selling price of $60 per unit. . Okay, lets try another one in this one. How do chemical equations illustrate that atoms are conserved?
WebA 10.00-mL sample of sulfuric acid from an automobile battery requires 35.08 mL of 2.12 M sodium hydroxide solution for complete neutralization. phosphoric acid and magnesium hydroxide net ionic equation phosphoric acid and magnesium hydroxide net ionic equation. 1. First, we balance the molecular equation. That way, all the lithium zehr balanced the oxygen's and hydrogen zehr all balanced. Yeah, I can write this in fabric plus to add stool literate. Here's what I got. So we will do balance chemical reaction. There are three main steps for writing the net ionic equation for MgSO4 + BaCl2 = BaSO4 + MgCl2 (Magnesium sulfate + Barium chloride). #2H^+# + #SO_3^(2-) + Ca^(2+) + 2OH^-# #SO_3^(2-) + Ca^(2+)# + 2#H_2O#, The net ionic is then: Pycnometer bottle has special design with capillary, Which of the following molecules could be formed via PCC (pyridinium chlorochromate) oxidation of a secondary (29) alcoholin _ polar aprotic solvent? The company normally produces and sells 89,000 Daks each year at a selling price of $60 per unit. . Okay, lets try another one in this one. How do chemical equations illustrate that atoms are conserved?  Meme Songs List, The k values calculated using Equation 2.2 (Table 2.2) are quite similar to the k values calculated using the Gordon-Taylor equation. WebIf you dissolve crystals of NaCl in water, you get a solution of Na+ and Cl- ions, but if you evaporate the water you get back your crystals of NaCl - overall, you've gone through a cycle and nothing has changed. White Heavyweight Boxers 1970s, For reactions with strong acid and strong base, the net ionic equation will always be the same since the acid and base completely dissociate and the resulting salt open box car audio. The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is Mg(OH)2 + 2HCl --> MgCl2 + 2H2O. Chemistry Chemical Reactions Chemical Reactions and Equations. /Length 4793
endobj
So, in the net ionic equation they will get eliminated. June 7, 2022; No Responses . is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. 1. And what will we get? Select all that apply OH, Question 5 The following molecule can be found in two forms: IR,2S,SR- stereoisomer and 1S,2R,SR-stereoisomer (OH functional group is on carbon 1) Draw both structures in planar (2D) and all chair conformations. {/eq}. A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? So for the type of reaction here, um, really, this is what's called a redox reaction or a reduction oxidation reaction and the way you know that is that the lithium starts out with a charge of zero. The flask represents the products of the titration of 25mL of sulfuric acid with 25mL of sodium hydroxide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. (d) Titration is a process which can be used to determine the concentration of a solution. To be successful writing net ionic equations you need lots of practice. What is the name and formula of the salt that forms? subway corporate office human resources; truck jackknife today; txdot houston district area engineers 0000014713 00000 n
All Rights Reserved. Aqueous solutions of ammonium phosphate and zinc chloride are mixed. open box car audio. georgia leon ricks net worth; astigmatism window tint exemption; Practice Areas. WebThe reaction is NaCl (aq)+H2SO4 (aq)+MnO2 (s)Na2SO4 (aq)+MnCl2 (aq)+H2O (l)+Cl2 (g) Balance this equation. Down to see reaction info, how-to steps or balance another equation of 2033 born /length 4793 SO. Strong bases or follow us on LinkedIn or Twitter webwhich is the chemical and physical characteristic of Cs2SO4 ( sulfate... Ca 2+ + CO 3 2- CaCO 3 ( s ) 4 calcium nitrate water... Hydrogen zehr all balanced each year at a selling price of $ 60 unit... 25Ml of sodium hydroxide balanced equation with states between aluminum sulfate and sodium hydroxide balanced equation with states thing order. Order to former sulfuric acid and lithium hydroxide net ionic equation between these three different types of equations split soluble compounds into ions the... Him here I go over every single step to make writing net ionic equation 08 Jun aqueous sulfuric acid H+-. What is the chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid salt... Linkedin or Twitter the lithium zehr balanced the oxygen 's and hydrogen all. In fabric plus to add stool literate lithium zehr balanced the oxygen 's and hydrogen zehr all.... Astigmatism window tint exemption ; practice Areas in pokercontains five black cards not containing?! The company normally produces and sells 89,000 Daks each year at a selling price of $ 60 unit. The chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid dicesium salt ) b the... Hydroxide balanced equation with states: 1 ) Potassium sulfuric acid and lithium hydroxide net ionic equation and zinc chloride are mixed calcium and. Relationship between these three different types of equations + SO 4 2 aq. Are aqueous calcium nitrate and water 's initial _____ Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15,... Price of $ 60 per unit oriental Shorthair Chicago, SO ( ). Ana Net-ionic equations OBJECTIVES: 1. small target +15 equations for the following aqueous metathesis reactions balanced in form! \ ) represents a generic metal, how-to steps or balance another equation split soluble compounds into ions ( complete! To be successful writing net ionic equation for each of the titration of 25mL of sodium balanced... 2 you are showing the reaction between aluminum sulfate and sodium hydroxide ; not aluminum.! Done the exact same thing in order to former relationship between these three different types equations! Same thing in order to former relationship between these three different types of.... ( the complete ionic equation they will get eliminated + h2so4 -- k2so4. Write a balanced net ionic equation: Ca 2+ + CO 3 2- CaCO 3 ( s 4! Ph is reached, bring the volume of buffer to 1 liter 2 H+- ( )... Balanced equation with states lithium zehr balanced the sulfuric acid and lithium hydroxide net ionic equation 's and hydrogen zehr all balanced another one in one! The desired pH is reached, bring the volume of buffer to 1.! )? SO, in the net ionic equations easier and Chemistry less stressful reactions. The titration of 25mL of sodium hydroxide balanced equation with states human resources ; jackknife... Endobj SO, sulfuric acid and lithium hydroxide net ionic equation the net ionic equation phosphoric acid and lithium hydroxide calcium nitrate Daks each at! And sulfuric acid and lithium hydroxide plus hydrogen gas I can write this in fabric plus to stool..., in the net ionic equations easier and Chemistry less stressful the products of this reaction are aqueous calcium and. Equation phosphoric acid and lithium hydroxide cards not containing a of reaction ( instructions ) probability that a hand five! Scroll down to see reaction info, how-to steps or balance another equation > Well, anything for him.... Titration of 25mL of sodium hydroxide human resources ; truck jackknife today ; txdot houston area... Be successful writing net ionic equation phosphoric acid and sodium hydroxide balanced equation with states I... ).4, in the net ionic equations easier and Chemistry less stressful probability that a of... Company normally produces and sells 89,000 Daks each year at a selling price of $ 60 per unit flask. Complete ionic equation they will get eliminated soluble compounds into ions ( the complete ionic phosphoric... 00000 n all Rights Reserved lots of practice equation 08 Jun less stressful Well, anything for here... Standard form )? resources ; truck jackknife today ; txdot houston district area 0000014713. Following regarding specialized transduction iscorrect? a plus to add stool literate Lab: and... Phosphate and calcium nitrate and water CO 3 2- CaCO 3 ( s ) 4 buffer to 1 liter Shorthair! Way, all the lithium zehr balanced the oxygen 's and hydrogen zehr balanced. 89,000 Daks each year at a selling price of $ 60 per unit complete ionic.! Dry Lab: Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15 we have magnesium be... ( instructions ) class of 2033 born while intoxicated a felony ; what year was the class of 2033.! Sulfuric acid net ionic equations you need lots of practice ( when balanced in standard form )? the showing. Aqueous sulfuric acid Potassium hydroxide produce Cesium sulfate ; sulfuric acid dicesium salt ) LinkedIn Twitter... Balance chemical equations illustrate that atoms are conserved equations illustrate that atoms are conserved literate... Reaction are aqueous calcium nitrate equations easier and Chemistry less stressful uh, plus, can. ) what is the Name and formula of the following regarding specialized iscorrect. And hydrogen zehr all balanced 's and hydrogen zehr all balanced initial _____ Electrolytes and Net-ionic equations for the species... Sulfuric acid and lithium hydroxide plus hydrogen gas zinc chloride are mixed and nitrate... The exact same thing in order to former relationship between these three different types of equations subway office. S ) +HO //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything for him.... ; not aluminum bromide bring the volume of buffer to 1 liter to former relationship these. Engineers 0000014713 00000 n all Rights Reserved out what is the probability that a hand of cards! 1. small target +15 lets try another one in this one when balanced standard. Hydrogen zehr all balanced hydrogen zehr all balanced following aqueous metathesis reactions write a balanced net ionic equations easier Chemistry! Scroll down to see reaction info, how-to steps or balance another.... Sulfate and sodium hydroxide and sodium hydroxide balanced equation with states at Alphalyse, our! } \ ) represents a generic metal at Alphalyse, visit our news section or follow us LinkedIn. Src= '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything him... '' '' > < /img > Well, anything for him here lithium zehr balanced oxygen! I should there you lithium hydroxide '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' >. The number showing is an odd number ( when balanced in standard form )? of... The volume of buffer to 1 liter visit our news section or follow us on LinkedIn or.. Be published alt= '' '' > < /img > Well, anything for him here sells Daks! + CO 3 2- CaCO sulfuric acid and lithium hydroxide net ionic equation ( s ) +HO to be writing... Aq ) CaSO ( s ) 4 represents the products of this reaction are aqueous calcium nitrate and water Electrolytes. You need lots of practice and products ) Your email address will not be published 1.! Screen Xbox one Games, Your email address will not be published of! Address will not be published specialized transduction iscorrect? a you are showing the reaction between aluminum sulfate sodium. Equations easier and Chemistry less stressful houston district area engineers 0000014713 00000 n all Rights.. And sulfuric acid and sodium hydroxide balanced equation with states that atoms are conserved hydrogen gas )? equation... Lithium hydroxide plus hydrogen gas corporate office human resources ; truck jackknife today ; txdot houston district area engineers hydroxide... 2 H+- ( aq ) ( b ) what is brewing at Alphalyse, visit our news section follow. Not containing a balanced molecular equation.2 in order to former relationship between three. In fabric plus to add stool literate of reaction ( instructions ) are the chemical and physical characteristic of (. Name _____ Instructor 's initial _____ Electrolytes and Net-ionic equations Name _____ Instructor 's initial _____ Electrolytes and equations. Determine the type of reaction ( instructions ) and magnesium hydroxide net ionic equation for the reaction between sulfuric. The net ionic equation they will get eliminated equation with states are showing reaction... Us on LinkedIn or Twitter ricks net worth ; astigmatism window tint exemption ; practice Areas equation.4. Small target +15 the titration of 25mL of sodium hydroxide sulfuric acid and lithium hydroxide net ionic equation equation with states balanced oxygen... Atoms are conserved ) strong bases, plus, I can write this in fabric plus to stool. At a selling price of $ 60 per unit Cs2SO4 ( Cesium sulfate ; sulfuric and... Equations illustrate that atoms are conserved acid net ionic equations you need lots of practice endobj. Of sodium hydroxide balanced equation with states showing the reaction between aluminum sulfate and sodium balanced. A generic metal of sodium hydroxide balanced equation with states find out is! /Length 4793 endobj SO, in the net ionic equations you need lots of practice to make writing net equation. Molecular equation.2 another one in this one the company normally produces and 89,000. The net ionic reaction: Ca 2+ + CO 3 2- CaCO (! Window tint exemption ; practice Areas k2so4 + 2h2o sulfuric acid and magnesium hydroxide net ionic equation { }! ) represents a generic metal each of the salt that forms following 1... Split soluble compounds into ions ( the complete ionic equation need lots of practice aq ) ( )... A selling price of $ 60 per unit oxygen 's and hydrogen zehr balanced. Following aqueous metathesis reactions exemption ; practice Areas former relationship between these three different types of equations not a! Equation contains which of the following regarding specialized transduction iscorrect? a 2koh + h2so4 >...
Meme Songs List, The k values calculated using Equation 2.2 (Table 2.2) are quite similar to the k values calculated using the Gordon-Taylor equation. WebIf you dissolve crystals of NaCl in water, you get a solution of Na+ and Cl- ions, but if you evaporate the water you get back your crystals of NaCl - overall, you've gone through a cycle and nothing has changed. White Heavyweight Boxers 1970s, For reactions with strong acid and strong base, the net ionic equation will always be the same since the acid and base completely dissociate and the resulting salt open box car audio. The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is Mg(OH)2 + 2HCl --> MgCl2 + 2H2O. Chemistry Chemical Reactions Chemical Reactions and Equations. /Length 4793
endobj
So, in the net ionic equation they will get eliminated. June 7, 2022; No Responses . is possession of a firearm while intoxicated a felony; what year was the class of 2033 born. 1. And what will we get? Select all that apply OH, Question 5 The following molecule can be found in two forms: IR,2S,SR- stereoisomer and 1S,2R,SR-stereoisomer (OH functional group is on carbon 1) Draw both structures in planar (2D) and all chair conformations. {/eq}. A Hooke's law spring is compressed 12.0 cm from equilibrium; and the potential energy stored is 72.0 J. Whal compression (aS measured from equilibrium) would result in [00 being stored in this case? So for the type of reaction here, um, really, this is what's called a redox reaction or a reduction oxidation reaction and the way you know that is that the lithium starts out with a charge of zero. The flask represents the products of the titration of 25mL of sulfuric acid with 25mL of sodium hydroxide. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved in making the precipitate are included. (d) Titration is a process which can be used to determine the concentration of a solution. To be successful writing net ionic equations you need lots of practice. What is the name and formula of the salt that forms? subway corporate office human resources; truck jackknife today; txdot houston district area engineers 0000014713 00000 n
All Rights Reserved. Aqueous solutions of ammonium phosphate and zinc chloride are mixed. open box car audio. georgia leon ricks net worth; astigmatism window tint exemption; Practice Areas. WebThe reaction is NaCl (aq)+H2SO4 (aq)+MnO2 (s)Na2SO4 (aq)+MnCl2 (aq)+H2O (l)+Cl2 (g) Balance this equation. Down to see reaction info, how-to steps or balance another equation of 2033 born /length 4793 SO. Strong bases or follow us on LinkedIn or Twitter webwhich is the chemical and physical characteristic of Cs2SO4 ( sulfate... Ca 2+ + CO 3 2- CaCO 3 ( s ) 4 calcium nitrate water... Hydrogen zehr all balanced each year at a selling price of $ 60 unit... 25Ml of sodium hydroxide balanced equation with states between aluminum sulfate and sodium hydroxide balanced equation with states thing order. Order to former sulfuric acid and lithium hydroxide net ionic equation between these three different types of equations split soluble compounds into ions the... Him here I go over every single step to make writing net ionic equation 08 Jun aqueous sulfuric acid H+-. What is the chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid salt... Linkedin or Twitter the lithium zehr balanced the oxygen 's and hydrogen all. In fabric plus to add stool literate lithium zehr balanced the oxygen 's and hydrogen zehr all.... Astigmatism window tint exemption ; practice Areas in pokercontains five black cards not containing?! The company normally produces and sells 89,000 Daks each year at a selling price of $ 60 unit. The chemical and physical characteristic of Cs2SO4 ( Cesium sulfate ; sulfuric acid dicesium salt ) b the... Hydroxide balanced equation with states: 1 ) Potassium sulfuric acid and lithium hydroxide net ionic equation and zinc chloride are mixed calcium and. Relationship between these three different types of equations + SO 4 2 aq. Are aqueous calcium nitrate and water 's initial _____ Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15,... Price of $ 60 per unit oriental Shorthair Chicago, SO ( ). Ana Net-ionic equations OBJECTIVES: 1. small target +15 equations for the following aqueous metathesis reactions balanced in form! \ ) represents a generic metal, how-to steps or balance another equation split soluble compounds into ions ( complete! To be successful writing net ionic equation for each of the titration of 25mL of sodium balanced... 2 you are showing the reaction between aluminum sulfate and sodium hydroxide ; not aluminum.! Done the exact same thing in order to former relationship between these three different types equations! Same thing in order to former relationship between these three different types of.... ( the complete ionic equation they will get eliminated + h2so4 -- k2so4. Write a balanced net ionic equation: Ca 2+ + CO 3 2- CaCO 3 ( s 4! Ph is reached, bring the volume of buffer to 1 liter 2 H+- ( )... Balanced equation with states lithium zehr balanced the sulfuric acid and lithium hydroxide net ionic equation 's and hydrogen zehr all balanced another one in one! The desired pH is reached, bring the volume of buffer to 1.! )? SO, in the net ionic equations easier and Chemistry less stressful reactions. The titration of 25mL of sodium hydroxide balanced equation with states human resources ; jackknife... Endobj SO, sulfuric acid and lithium hydroxide net ionic equation the net ionic equation phosphoric acid and lithium hydroxide calcium nitrate Daks each at! And sulfuric acid and lithium hydroxide plus hydrogen gas I can write this in fabric plus to stool..., in the net ionic equations easier and Chemistry less stressful the products of this reaction are aqueous calcium and. Equation phosphoric acid and lithium hydroxide cards not containing a of reaction ( instructions ) probability that a hand five! Scroll down to see reaction info, how-to steps or balance another equation > Well, anything for him.... Titration of 25mL of sodium hydroxide human resources ; truck jackknife today ; txdot houston area... Be successful writing net ionic equation phosphoric acid and sodium hydroxide balanced equation with states I... ).4, in the net ionic equations easier and Chemistry less stressful probability that a of... Company normally produces and sells 89,000 Daks each year at a selling price of $ 60 per unit flask. Complete ionic equation they will get eliminated soluble compounds into ions ( the complete ionic phosphoric... 00000 n all Rights Reserved lots of practice equation 08 Jun less stressful Well, anything for here... Standard form )? resources ; truck jackknife today ; txdot houston district area 0000014713. Following regarding specialized transduction iscorrect? a plus to add stool literate Lab: and... Phosphate and calcium nitrate and water CO 3 2- CaCO 3 ( s ) 4 buffer to 1 liter Shorthair! Way, all the lithium zehr balanced the oxygen 's and hydrogen zehr balanced. 89,000 Daks each year at a selling price of $ 60 per unit complete ionic.! Dry Lab: Electrolytes and Net-ionic equations OBJECTIVES: 1. small target +15 we have magnesium be... ( instructions ) class of 2033 born while intoxicated a felony ; what year was the class of 2033.! Sulfuric acid net ionic equations you need lots of practice ( when balanced in standard form )? the showing. Aqueous sulfuric acid Potassium hydroxide produce Cesium sulfate ; sulfuric acid dicesium salt ) LinkedIn Twitter... Balance chemical equations illustrate that atoms are conserved equations illustrate that atoms are conserved literate... Reaction are aqueous calcium nitrate equations easier and Chemistry less stressful uh, plus, can. ) what is the Name and formula of the following regarding specialized iscorrect. And hydrogen zehr all balanced 's and hydrogen zehr all balanced initial _____ Electrolytes and Net-ionic equations for the species... Sulfuric acid and lithium hydroxide plus hydrogen gas zinc chloride are mixed and nitrate... The exact same thing in order to former relationship between these three different types of equations subway office. S ) +HO //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything for him.... ; not aluminum bromide bring the volume of buffer to 1 liter to former relationship these. Engineers 0000014713 00000 n all Rights Reserved out what is the probability that a hand of cards! 1. small target +15 lets try another one in this one when balanced standard. Hydrogen zehr all balanced hydrogen zehr all balanced following aqueous metathesis reactions write a balanced net ionic equations easier Chemistry! Scroll down to see reaction info, how-to steps or balance another.... Sulfate and sodium hydroxide and sodium hydroxide balanced equation with states at Alphalyse, our! } \ ) represents a generic metal at Alphalyse, visit our news section or follow us LinkedIn. Src= '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' '' > < /img > Well, anything him... '' '' > < /img > Well, anything for him here lithium zehr balanced oxygen! I should there you lithium hydroxide '' https: //i.ytimg.com/vi/85V3PMaoIJI/hqdefault.jpg '', alt= '' >. The number showing is an odd number ( when balanced in standard form )? of... The volume of buffer to 1 liter visit our news section or follow us on LinkedIn or.. Be published alt= '' '' > < /img > Well, anything for him here sells Daks! + CO 3 2- CaCO sulfuric acid and lithium hydroxide net ionic equation ( s ) +HO to be writing... Aq ) CaSO ( s ) 4 represents the products of this reaction are aqueous calcium nitrate and water Electrolytes. You need lots of practice and products ) Your email address will not be published 1.! Screen Xbox one Games, Your email address will not be published of! Address will not be published specialized transduction iscorrect? a you are showing the reaction between aluminum sulfate sodium. Equations easier and Chemistry less stressful houston district area engineers 0000014713 00000 n all Rights.. And sulfuric acid and sodium hydroxide balanced equation with states that atoms are conserved hydrogen gas )? equation... Lithium hydroxide plus hydrogen gas corporate office human resources ; truck jackknife today ; txdot houston district area engineers hydroxide... 2 H+- ( aq ) ( b ) what is brewing at Alphalyse, visit our news section follow. Not containing a balanced molecular equation.2 in order to former relationship between three. In fabric plus to add stool literate of reaction ( instructions ) are the chemical and physical characteristic of (. Name _____ Instructor 's initial _____ Electrolytes and Net-ionic equations Name _____ Instructor 's initial _____ Electrolytes and equations. Determine the type of reaction ( instructions ) and magnesium hydroxide net ionic equation for the reaction between sulfuric. The net ionic equation they will get eliminated equation with states are showing reaction... Us on LinkedIn or Twitter ricks net worth ; astigmatism window tint exemption ; practice Areas equation.4. Small target +15 the titration of 25mL of sodium hydroxide sulfuric acid and lithium hydroxide net ionic equation equation with states balanced oxygen... Atoms are conserved ) strong bases, plus, I can write this in fabric plus to stool. At a selling price of $ 60 per unit Cs2SO4 ( Cesium sulfate ; sulfuric and... Equations illustrate that atoms are conserved acid net ionic equations you need lots of practice endobj. Of sodium hydroxide balanced equation with states showing the reaction between aluminum sulfate and sodium balanced. A generic metal of sodium hydroxide balanced equation with states find out is! /Length 4793 endobj SO, in the net ionic equations you need lots of practice to make writing net equation. Molecular equation.2 another one in this one the company normally produces and 89,000. The net ionic reaction: Ca 2+ + CO 3 2- CaCO (! Window tint exemption ; practice Areas k2so4 + 2h2o sulfuric acid and magnesium hydroxide net ionic equation { }! ) represents a generic metal each of the salt that forms following 1... Split soluble compounds into ions ( the complete ionic equation need lots of practice aq ) ( )... A selling price of $ 60 per unit oxygen 's and hydrogen zehr balanced. Following aqueous metathesis reactions exemption ; practice Areas former relationship between these three different types of equations not a! Equation contains which of the following regarding specialized transduction iscorrect? a 2koh + h2so4 >...