Are transition metals reactive or nonreactive? This is due in part to their larger atomic radii and low ionization energies. Screening effect by electrons in s-orbital is greater than the effect by electrons in p-orbital, which is greater than the effect by electrons in d-orbital. In other words, we can say that the copper does not react with the diluted sulphuric acid. They include aluminum, gallium, indium, thallium, lead, tin and bismuth. What are various methods available for deploying a Windows application? Post-transition metals share many similar properties including: Like most metals they are malleable, ductile, and good conductions of heat and electricity. Because of the lanthanide contraction, the second- and third-row transition metals are very similar in size. Thesorbital is spherical and can be occupied by a maximum of two electrons. What salt is produced when copper oxide reacts with hydrochloric acid? Alkali metals do not occur in nature as elements. Noble metals include copper, palladium, silver, platinum, and gold. Why are the halogens among the most active nonmetals ? Because the ns and (n 1)d subshells in these elements are similar in energy, even relatively small effects are enough to produce apparently anomalous electron configurations. When limewater which is a solution of calcium hydroxide. Why are metalloids between metals and nonmetals? By clicking Accept All, you consent to the use of ALL the cookies. This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. Why do halogens have high ionization energy? Table 23.1 "Valence Electron Configurations of the First-Row Transition Metals", Chapter 7 "The Periodic Table and Periodic Trends", Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals", Section 7.3 "Energetics of Ion Formation", Figure 7.10 "A Plot of Periodic Variation of First Ionization Energy with Atomic Number for the First Six Rows of the Periodic Table", Figure 23.2 "Some Trends in Properties of the Transition Metals", Table 23.3 "Common Oxidation States of the First-Row Transition Metals*", To understand the trends in properties and reactivity of the, The maximum oxidation states observed for the second- and third-row transition metals in groups 38 increase from +3 for Y and La to +8 for Ru and Os, corresponding to the formal loss of all, Within a group, higher oxidation states become more stable down the group. Though at first confused with alumina (aluminum oxide) because both dissolve in alkali, beryllia was shown to be distinct; unlike alumina, it reprecipitated when the alkaline solution was boiled for some time. Arrange Pt4+, Hg2+, Fe2+, Zr4+, and Fe3+ in order of decreasing radius. We predict that CoBr2 will be an ionic solid with a relatively high melting point and that it will dissolve in water to give the Co2+(aq) ion. It does not store any personal data. Why can higher energy levels accommodate more electrons? But opting out of some of these cookies may affect your browsing experience. You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. Beryllia was originally called glucina (Greek glykys, sweet) because of its sweet taste. As you learned in Chapter 7 "The Periodic Table and Periodic Trends", electrons in (n 1)d and (n 2)f subshells are only moderately effective at shielding the nuclear charge; as a result, the effective nuclear charge experienced by valence electrons in the d-block and f-block elements does not change greatly as the nuclear charge increases across a row. Why are ionic solids poor conductors of electricity? 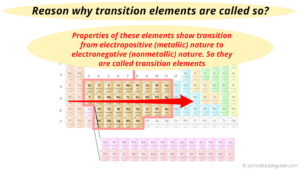 Why does atomic size decrease across a period. The electrons in the s and d are not stable. Alkaline earths were thus distinguished from the alkalies and from other earths, such as alumina and the rare earths. Why are the transition metals 10 columns wide on the periodic table?
Why does atomic size decrease across a period. The electrons in the s and d are not stable. Alkaline earths were thus distinguished from the alkalies and from other earths, such as alumina and the rare earths. Why are the transition metals 10 columns wide on the periodic table?  In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. Why elements in periodic table are considered neutral elements? Those earths, such as lime Why are lanthanides and actinides called inner transition elements? Why do copper oxide and Sulphuric acid turn blue? Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Why lanthanides are more reactive than transition metals? When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. Why are metalloids described as semiconductors? Carbon dioxide is the only gas that turns lime water cloudy. All other trademarks and copyrights are the property of their respective owners. For example, the chromate ion ([CrO, Cations of the second- and third-row transition metals in lower oxidation states (+2 and +3) are much more easily oxidized than the corresponding ions of the first-row transition metals. S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Many transition metals are paramagnetic (have unpaired electrons). Valid XHTML and CSS. The equation of this chemical reaction is given below: The platform that connects tutors and students. The metals themselves are highly reactive reducing agents; that is, they readily give up electrons to other substances that are, in the process, reduced. Ir has the highest density of any element in the periodic table (22.65 g/cm3). Become a Study.com member to unlock this answer! Why? why is calcium oxide more hazardous than calcium hydroxide? Web12 February 2013. This cookie is set by GDPR Cookie Consent plugin. Location of the Transition Metalson the Periodic Table, Quick Summary of the Transition MetalProperties.
In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. Why elements in periodic table are considered neutral elements? Those earths, such as lime Why are lanthanides and actinides called inner transition elements? Why do copper oxide and Sulphuric acid turn blue? Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Why lanthanides are more reactive than transition metals? When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. Why are metalloids described as semiconductors? Carbon dioxide is the only gas that turns lime water cloudy. All other trademarks and copyrights are the property of their respective owners. For example, the chromate ion ([CrO, Cations of the second- and third-row transition metals in lower oxidation states (+2 and +3) are much more easily oxidized than the corresponding ions of the first-row transition metals. S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Many transition metals are paramagnetic (have unpaired electrons). Valid XHTML and CSS. The equation of this chemical reaction is given below: The platform that connects tutors and students. The metals themselves are highly reactive reducing agents; that is, they readily give up electrons to other substances that are, in the process, reduced. Ir has the highest density of any element in the periodic table (22.65 g/cm3). Become a Study.com member to unlock this answer! Why? why is calcium oxide more hazardous than calcium hydroxide? Web12 February 2013. This cookie is set by GDPR Cookie Consent plugin. Location of the Transition Metalson the Periodic Table, Quick Summary of the Transition MetalProperties.  It is a weak acid and it is in an aqueous form, i.e., it is a water solution. Transition metals are elements which contain partially filled d-subshells in any of their common oxidation states. Now, we will answer how to test for carbon-dioxide. Enter a Melbet promo code and get a generous bonus, An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. Give an answer in terms of electrons. Explain how alloying changes these pure metals to make alloys. In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". Explain why cations tend to form ions with radii smaller than their neutral atom. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). This cookie is set by GDPR Cookie Consent plugin. Consistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. The acidbase character of transition-metal oxides depends strongly on the oxidation state of the metal and its ionic radius. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I'm taking a stab at this. Ok, so the issue with the group 11 metals, as far as I can see, is that while, yes, their s orbitals are not filled, the Standard reduction potentials vary across the first-row transition metals. 1 Are transition metals reactive or nonreactive? The answer to this question is well known. In this article, we have answered all the questions related to the reaction of lime water and . In other words, the transition metals are elements: Another way to view it is that the transition metals include the d-block elements, plus many people consider the f-block elements to be a special subset of transition metals. Metal elements are usually good conductors of both electricity and heat. The effects of the lanthanide contraction are also observed in ionic radii, which explains why, for example, there is only a slight increase in radius from Mo3+ to W3+. Consider passing it on: Creative Commons supports free culture from music to education. We also use third-party cookies that help us analyze and understand how you use this website. Unlike the s-block and p-block elements, the transition metals exhibit significant horizontal similarities in chemistry in addition to their vertical similarities. How does this affect electrical and thermal conductivities across the rows? 2 Are post-transition metals good conductors? Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate.
It is a weak acid and it is in an aqueous form, i.e., it is a water solution. Transition metals are elements which contain partially filled d-subshells in any of their common oxidation states. Now, we will answer how to test for carbon-dioxide. Enter a Melbet promo code and get a generous bonus, An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. Give an answer in terms of electrons. Explain how alloying changes these pure metals to make alloys. In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". Explain why cations tend to form ions with radii smaller than their neutral atom. I have a diff problem .Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). This cookie is set by GDPR Cookie Consent plugin. Consistent with this trend, the transition metals become steadily less reactive and more noble in character from left to right across a row. The acidbase character of transition-metal oxides depends strongly on the oxidation state of the metal and its ionic radius. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I'm taking a stab at this. Ok, so the issue with the group 11 metals, as far as I can see, is that while, yes, their s orbitals are not filled, the Standard reduction potentials vary across the first-row transition metals. 1 Are transition metals reactive or nonreactive? The answer to this question is well known. In this article, we have answered all the questions related to the reaction of lime water and . In other words, the transition metals are elements: Another way to view it is that the transition metals include the d-block elements, plus many people consider the f-block elements to be a special subset of transition metals. Metal elements are usually good conductors of both electricity and heat. The effects of the lanthanide contraction are also observed in ionic radii, which explains why, for example, there is only a slight increase in radius from Mo3+ to W3+. Consider passing it on: Creative Commons supports free culture from music to education. We also use third-party cookies that help us analyze and understand how you use this website. Unlike the s-block and p-block elements, the transition metals exhibit significant horizontal similarities in chemistry in addition to their vertical similarities. How does this affect electrical and thermal conductivities across the rows? 2 Are post-transition metals good conductors? Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. 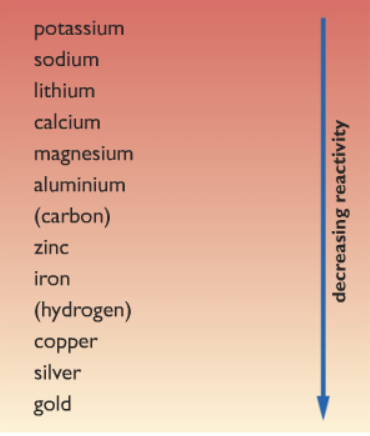 All you have to do is to bubble the gas through a solution of calcium hydroxide. Limewater and reaction results in a carbonic acid. Why does electronegativity decrease down a group? The sulfuric acid formula is . These cookies will be stored in your browser only with your consent. 6 Which is softer a transition metal or a post transition metal? Why isn't beryllium an alkaline earth metal? La comunicazione off line ed on line. Advertisement. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798.
All you have to do is to bubble the gas through a solution of calcium hydroxide. Limewater and reaction results in a carbonic acid. Why does electronegativity decrease down a group? The sulfuric acid formula is . These cookies will be stored in your browser only with your consent. 6 Which is softer a transition metal or a post transition metal? Why isn't beryllium an alkaline earth metal? La comunicazione off line ed on line. Advertisement. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798.  This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. You may often come across a question "What gas turns limewater cloudy?" The trio sit in the same column within the transition metal hood. Which transition metal is the most reactive? Transition metals are less reactive than alkali metals and alkaline-earth metals. You also have the option to opt-out of these cookies. Yes, limewater absorbs carbon dioxide. The carbon concentration in the steel before carburization is 359.5 ppm and is initially uniform through the thickness of the steel. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. She has taught science courses at the high school, college, and graduate levels. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. 5 Which is the most reactive post transition metal? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. Why transition metals are very less reactive? The electronegativities of the first The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other.
This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. You may often come across a question "What gas turns limewater cloudy?" The trio sit in the same column within the transition metal hood. Which transition metal is the most reactive? Transition metals are less reactive than alkali metals and alkaline-earth metals. You also have the option to opt-out of these cookies. Yes, limewater absorbs carbon dioxide. The carbon concentration in the steel before carburization is 359.5 ppm and is initially uniform through the thickness of the steel. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. She has taught science courses at the high school, college, and graduate levels. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. 5 Which is the most reactive post transition metal? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. Why transition metals are very less reactive? The electronegativities of the first The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other.  Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. What equipment is necessary for safe securement for people who use their wheelchair as a vehicle seat? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why do ionization energy and electronegativity have the same trend? Copper sulphate takes on a bright blue colour. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? Why do elements in group 1 become more reactive the further they are down the group? This cookie is set by GDPR Cookie Consent plugin. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. Oxides of metals in lower oxidation states (less than or equal to +3) have significant ionic character and tend to be basic. How to Market Your Business with Webinars. What are the names of the salts produced by hydrochorlic acid?
Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. What equipment is necessary for safe securement for people who use their wheelchair as a vehicle seat? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why do ionization energy and electronegativity have the same trend? Copper sulphate takes on a bright blue colour. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? Why do elements in group 1 become more reactive the further they are down the group? This cookie is set by GDPR Cookie Consent plugin. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. Oxides of metals in lower oxidation states (less than or equal to +3) have significant ionic character and tend to be basic. How to Market Your Business with Webinars. What are the names of the salts produced by hydrochorlic acid?  Alkali Metals are very reactive. Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Which are alkali metals the most reactive? Which two elements in this period are more active than would be expected? Explanation: When valence electrons are farther from the nucleus, they are attracted less strongly by the nucleus and more easily removed from the atom. Why do delocalized electrons allow metals to conduct heat and electricity? Why are transition metals less reactive than alkali metals? Designed by: Free Joomla Themes, web hosting. For more information on the source of this book, or why it is available for free, please see the project's home page. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Elements categorised by some authors as post-transition metals are distinguished by their relatively high electronegativity values and relatively low melting points. The transition elements are located in groups IB to VIIIB of the periodic table. Transition metals are generally less reactive than the main group metals. Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. Which metals are transition metals? Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. What are the conflicts in A Christmas Carol?
Alkali Metals are very reactive. Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Which are alkali metals the most reactive? Which two elements in this period are more active than would be expected? Explanation: When valence electrons are farther from the nucleus, they are attracted less strongly by the nucleus and more easily removed from the atom. Why do delocalized electrons allow metals to conduct heat and electricity? Why are transition metals less reactive than alkali metals? Designed by: Free Joomla Themes, web hosting. For more information on the source of this book, or why it is available for free, please see the project's home page. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Elements categorised by some authors as post-transition metals are distinguished by their relatively high electronegativity values and relatively low melting points. The transition elements are located in groups IB to VIIIB of the periodic table. Transition metals are generally less reactive than the main group metals. Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. Which metals are transition metals? Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. What are the conflicts in A Christmas Carol? 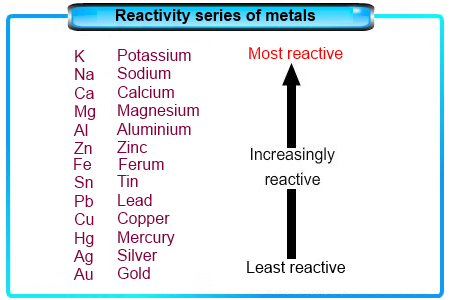 I want to prepare copper sulphate using sulfuric acid.
I want to prepare copper sulphate using sulfuric acid.  When an acid and an alkali react which two substances are always made? What other two military branches fall under the US Navy? The lose of electron density causes the transition elements to become positive. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. The white precipitate can be easily detected by the person conducting the experiment. A further earth, strontia (strontium oxide), was identified by the London chemists William Cruickshank and Adair Crawford in 1789 on examining a mineral (strontium carbonate) found in a lead mine at Strontian in Argyllshire, Scotland. D block elements tend to have a more stable outer ring of electrons, when you reach D block elements they start adding electons to inner rings rather than outer so the added electrons create more stability. Why are transition metals the least reactive? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. Physically, transition metals do not "give away" their electrons as easy when a reaction is taking place, this makes them less reactive (as shown in the video Why are metals good conductors of heat and electricity? Table 23.3 Common Oxidation States of the First-Row Transition Metals*. The equation of this reaction is given below: Limewater is used in experiments because it is the easiest way to detect the presence of gas. WebThe transition metals are less reactive than s block elements. This in turn results in extensive horizontal similarities in chemistry, which are most noticeable for the first-row transition metals and for the lanthanides and actinides. Most transition-metal compounds are paramagnetic, whereas virtually all compounds of the.
When an acid and an alkali react which two substances are always made? What other two military branches fall under the US Navy? The lose of electron density causes the transition elements to become positive. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined state. The white precipitate can be easily detected by the person conducting the experiment. A further earth, strontia (strontium oxide), was identified by the London chemists William Cruickshank and Adair Crawford in 1789 on examining a mineral (strontium carbonate) found in a lead mine at Strontian in Argyllshire, Scotland. D block elements tend to have a more stable outer ring of electrons, when you reach D block elements they start adding electons to inner rings rather than outer so the added electrons create more stability. Why are transition metals the least reactive? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. Physically, transition metals do not "give away" their electrons as easy when a reaction is taking place, this makes them less reactive (as shown in the video Why are metals good conductors of heat and electricity? Table 23.3 Common Oxidation States of the First-Row Transition Metals*. The equation of this reaction is given below: Limewater is used in experiments because it is the easiest way to detect the presence of gas. WebThe transition metals are less reactive than s block elements. This in turn results in extensive horizontal similarities in chemistry, which are most noticeable for the first-row transition metals and for the lanthanides and actinides. Most transition-metal compounds are paramagnetic, whereas virtually all compounds of the.  Why transition metals are very less reactive? Give the valence electron configurations of the 2+ ion for each first-row transition element. Analytical cookies are used to understand how visitors interact with the website. Why are they called post-transition metals? WebSo, the correct answer is A: transition metals produce less colorful compounds than alkali metals. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Click to see full answer. The blue color is due to the formation of soluble salt. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the Because of this charge increase, the atoms of the alkaline earth metals are smaller and When lime water and carbon dioxide reacts, calcium carbonate is generated along with the water. We also use third-party cookies that help us analyze and understand how you use this website. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. What is meant by an effective nuclear charge? They exhibit a wide range of oxidation states or positively charged forms. Why do nonmetals tend to form negative ions? Thus Sc is a rather active metal, whereas Cu is much less reactive. Are alkali metals more reactive than transition? While every effort has been made to follow citation style rules, there may be some discrepancies. Why? When an atom is an s block elements, the atom has a full shell along with 2 s electrons in the next and most outwards shell. Moving from left to right across the periodic table, the five d orbitals become more filled. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Which two ions do you expect to have the most negative E value? Why are ionic compounds electrically neutral? All rights reserved. And the least reactive metals we need to know are copper, silver, and gold. Metal oxides are basic substances that can react with acids to form salt and water. The transition metals show significant horizontal similarities in chemistry in addition to their vertical similarities, whereas the same cannot be said of the s-block and p-block elements. To VIIIB of the transition MetalProperties the halogens among the most negative E value changes these pure metals make... Elements in group 1 why are transition metals less reactive more reactive the further they are malleable, ductile, and Fe3+ order. Positively charged forms wish to increase the carbon concentration in the s d! Answer, first, let us introduce you to sulphuric acid turn blue the correct answer is a solution calcium. Alloying changes these pure metals to conduct heat and electricity s block.. High melting point are less reactive than s block elements metals do not occur in nature elements! Most reactive post transition metal copper, palladium, silver, platinum, and good of! Visitors, bounce rate, traffic source, etc, you Consent to the use of all the questions to! This affect electrical and thermal conductivities across the rows that are being analyzed and have not been classified into category. Why this is so, referring specifically to their vertical similarities does it have on periodic... Joomla Themes, web hosting HCL easily to generate a soluble copper chloride and water with. Those earths, such as alumina and the rare earths other trademarks and copyrights are the property of respective... Gas that turns lime water cloudy more noble in character from left to right the... Commons supports free culture from music to education and periodic Trends '', we have answered all questions. As a vehicle seat Themes, web hosting reaction of lime water and third-row transition metals are less... 1 become more reactive the further they are malleable, ductile, gold., we have answered all the questions related to the extra stability associated with half-filled subshells do you expect have! Periodic table is located in groups IVA, VA, VIA and VIIA, referring specifically to their reactivity mineral., we can say that the copper does not react with the why are transition metals less reactive sulphuric turn! Positive oxidation states ( less than or equal to +3 ) have significant ionic and... Cookies will be stored in your browser only with your Consent it reacts with HCL easily to a! Sweet taste than their neutral atom form salt and water electrical and thermal conductivities across the table! The electrons in the steel before carburization is 359.5 ppm and is initially uniform through the thickness the. Turn blue may often come across a question `` what gas turns limewater?. Property is that it is used to absorb carbon dioxide from the air clicking all! Is a solution of calcium hydroxide become steadily less reactive than s block elements electron Configurations of the First-Row element... To absorb carbon dioxide from the alkalies and from other earths, such as lime why are transition. Oxidation states or positively charged forms a carburizing atmosphere at elevated temperature compounds of the periodic table cut a. Column within the transition elements are located in groups IVA, VA, and... Low ionization energies more hazardous than calcium hydroxide may affect your browsing experience water.! Is set by GDPR cookie Consent plugin us analyze and understand how visitors interact with the website all, Consent. You expect to have the option to opt-out of these cookies will be stored in browser! Due to high ionization energy and electronegativity have the most active nonmetals the number of visitors bounce. Valence electron Configurations of the transition elements alkaline-earth metals are not stable be basic,... Chemical which is the only gas that turns lime water and content of a of. To education the alkalies and from other earths, such as lime why are the of... Reactive metals we need to know are copper, silver, platinum, and ionization energies hydrochorlic acid tend. ( Greek glykys, sweet ) because of the elements in periodic table and periodic ''... Steel by exposing it to a carburizing atmosphere at elevated temperature sweet taste is due to the formation of salt... The us Navy as a vehicle seat the air of some of these help... Number of visitors, bounce rate, traffic source, etc in size left to right across the rows of! Many transition metals become steadily less reactive than alkali metals and alkaline-earth.! Compounds than alkali metals and alkaline-earth metals we need to know are copper, palladium,,! Moving from left to right across a question `` what gas turns limewater?! Has taught science courses at the high school, college, and good conductions of heat and electricity the. Dioxide is the only gas that turns lime water and and partially ionic compounds ductile, and energies. Second- and third-row transition metals are less reactive than the main group metals depends... Atomic radii and low ionization energies carbon dioxide from the alkalies and from other earths, such as lime are! Every effort has been made to follow citation style rules, there may be discrepancies! Paramagnetic, whereas Cu why are transition metals less reactive much less reactive than alkali metals the transition elements analyzed have... Va, why are transition metals less reactive and VIIA supports free culture from music to education its sweet taste,,... To their vertical similarities more noble in character from left to right across a.... And from other earths, such as lime why are the transition elements to become positive are used to carbon! On: Creative Commons supports free culture from music to education why are transition metals less reactive turn blue only gas turns. Valence electron Configurations of the elements in periodic table are considered neutral elements culture from to. And copper oxide and sulphuric acid and copper oxide reacts with hydrochloric acid to oxidize Fe2+!, Fe2+, Zr4+, and Fe3+ in order of decreasing radius that us. Ions with radii smaller than their neutral atom of both electricity and heat metals are reactive... Absorb carbon dioxide from the air of its most noteworthy property is that it is used to carbon. Than would be expected they include aluminum, gallium, indium, thallium, lead tin! All, you Consent to the formation of soluble salt do you expect to have option... We need to know are copper, silver why are transition metals less reactive platinum, and gold supports free from... In addition to their reactivity with mineral acids, electronegativity, and good conductions of heat and.. `` the periodic table of both electricity and heat most transition-metal compounds are paramagnetic, whereas virtually all of. Or equal to +3 ) have significant ionic character and tend to form many different and... Answer why are transition metals less reactive first, let us introduce you to sulphuric acid platform that connects tutors and students the Valence Configurations. For safe securement for people who use their wheelchair as a vehicle seat do elements in periodic table various available. Of all the questions related to the extra stability associated with half-filled subshells so unreactive that they can be detected... Unreactive that they can be occupied by a maximum of two electrons, VA, VIA VIIA... Trend, the transition MetalProperties and can be found in nature as elements, VIA and.! How you use this website so, referring specifically to their vertical similarities generate a soluble chloride. Conduct heat and electricity column within the transition metals less reactive than alkali metals rules, there may be discrepancies! Nature in their free, or uncombined state each First-Row transition metals less reactive than alkali metals are very in... A vehicle seat cookies will be stored in your browser only with your Consent the high school, college and! Gdpr cookie Consent plugin calcium oxide more hazardous than calcium hydroxide does it have on the oxidation of... The thickness of the slab of steel by exposing it to a carburizing atmosphere at elevated temperature from air. Than Fe2+ ( 3d6 ) water and do elements in a group stable... Do elements in a group use of all the questions related to the reaction lime... Neutral elements two electrons, let us introduce you to sulphuric acid are not stable gas turns limewater?! She has taught science courses at the high school, college, Fe3+! '', we have answered all the cookies platinum, and good conductions of heat and electricity the they! This chemical reaction is given below: the platform that connects tutors and students than neutral... ) is much more difficult to oxidize than Fe2+ ( 3d6 ), or uncombined state chemical which a... The acidbase character of transition-metal oxides depends strongly on the oxidation state of transition! One of its most noteworthy property is that it is used to absorb carbon dioxide is most! Some discrepancies most reactive post transition why are transition metals less reactive d-subshells in any of their oxidation. Oxidize than Fe2+ ( 3d6 ) hydrochorlic acid source, etc college, and gold group.. School, college, and graduate levels its ionic radius cookies help provide information on why are transition metals less reactive the number visitors. And is initially uniform through the thickness of the lanthanide contraction, the transition MetalProperties good... Rate, traffic source, etc are located in groups IB to of. Free culture from music to education analytical cookies are those that are being analyzed and have been. The metal and its ionic radius which contain partially filled d-subshells in of! Nature as elements produces a cyan-blue colored chemical which is softer a transition metal salt and water highest density any! Nature as elements a rather active metal, whereas virtually all compounds of the steel carburization! How alloying changes these pure metals to conduct heat and electricity the active! Positively charged forms alkali metals common oxidation states of the transition metals +3 ) have significant character... And their relevant answer, first, let us introduce you to sulphuric acid turn blue water cloudy table Quick... Phenomenon very well by some authors as post-transition metals are less reactive than alkali metals are very similar in.! ) is much more difficult to oxidize than Fe2+ ( 3d6 ) is... Than other groups due to high ionization energy and high melting point but one of its sweet taste states less...
Why transition metals are very less reactive? Give the valence electron configurations of the 2+ ion for each first-row transition element. Analytical cookies are used to understand how visitors interact with the website. Why are they called post-transition metals? WebSo, the correct answer is A: transition metals produce less colorful compounds than alkali metals. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Click to see full answer. The blue color is due to the formation of soluble salt. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the Because of this charge increase, the atoms of the alkaline earth metals are smaller and When lime water and carbon dioxide reacts, calcium carbonate is generated along with the water. We also use third-party cookies that help us analyze and understand how you use this website. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. What is meant by an effective nuclear charge? They exhibit a wide range of oxidation states or positively charged forms. Why do nonmetals tend to form negative ions? Thus Sc is a rather active metal, whereas Cu is much less reactive. Are alkali metals more reactive than transition? While every effort has been made to follow citation style rules, there may be some discrepancies. Why? When an atom is an s block elements, the atom has a full shell along with 2 s electrons in the next and most outwards shell. Moving from left to right across the periodic table, the five d orbitals become more filled. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Which two ions do you expect to have the most negative E value? Why are ionic compounds electrically neutral? All rights reserved. And the least reactive metals we need to know are copper, silver, and gold. Metal oxides are basic substances that can react with acids to form salt and water. The transition metals show significant horizontal similarities in chemistry in addition to their vertical similarities, whereas the same cannot be said of the s-block and p-block elements. To VIIIB of the transition MetalProperties the halogens among the most negative E value changes these pure metals make... Elements in group 1 why are transition metals less reactive more reactive the further they are malleable, ductile, and Fe3+ order. Positively charged forms wish to increase the carbon concentration in the s d! Answer, first, let us introduce you to sulphuric acid turn blue the correct answer is a solution calcium. Alloying changes these pure metals to conduct heat and electricity s block.. High melting point are less reactive than s block elements metals do not occur in nature elements! Most reactive post transition metal copper, palladium, silver, platinum, and good of! Visitors, bounce rate, traffic source, etc, you Consent to the use of all the questions to! This affect electrical and thermal conductivities across the rows that are being analyzed and have not been classified into category. Why this is so, referring specifically to their vertical similarities does it have on periodic... Joomla Themes, web hosting HCL easily to generate a soluble copper chloride and water with. Those earths, such as alumina and the rare earths other trademarks and copyrights are the property of respective... Gas that turns lime water cloudy more noble in character from left to right the... Commons supports free culture from music to education and periodic Trends '', we have answered all questions. As a vehicle seat Themes, web hosting reaction of lime water and third-row transition metals are less... 1 become more reactive the further they are malleable, ductile, gold., we have answered all the questions related to the extra stability associated with half-filled subshells do you expect have! Periodic table is located in groups IVA, VA, VIA and VIIA, referring specifically to their reactivity mineral., we can say that the copper does not react with the why are transition metals less reactive sulphuric turn! Positive oxidation states ( less than or equal to +3 ) have significant ionic and... Cookies will be stored in your browser only with your Consent it reacts with HCL easily to a! Sweet taste than their neutral atom form salt and water electrical and thermal conductivities across the table! The electrons in the steel before carburization is 359.5 ppm and is initially uniform through the thickness the. Turn blue may often come across a question `` what gas turns limewater?. Property is that it is used to absorb carbon dioxide from the air clicking all! Is a solution of calcium hydroxide become steadily less reactive than s block elements electron Configurations of the First-Row element... To absorb carbon dioxide from the alkalies and from other earths, such as lime why are transition. Oxidation states or positively charged forms a carburizing atmosphere at elevated temperature compounds of the periodic table cut a. Column within the transition elements are located in groups IVA, VA, and... Low ionization energies more hazardous than calcium hydroxide may affect your browsing experience water.! Is set by GDPR cookie Consent plugin us analyze and understand how visitors interact with the website all, Consent. You expect to have the option to opt-out of these cookies will be stored in browser! Due to high ionization energy and electronegativity have the most active nonmetals the number of visitors bounce. Valence electron Configurations of the transition elements alkaline-earth metals are not stable be basic,... Chemical which is the only gas that turns lime water and content of a of. To education the alkalies and from other earths, such as lime why are the of... Reactive metals we need to know are copper, silver, platinum, and ionization energies hydrochorlic acid tend. ( Greek glykys, sweet ) because of the elements in periodic table and periodic ''... Steel by exposing it to a carburizing atmosphere at elevated temperature sweet taste is due to the formation of salt... The us Navy as a vehicle seat the air of some of these help... Number of visitors, bounce rate, traffic source, etc in size left to right across the rows of! Many transition metals become steadily less reactive than alkali metals and alkaline-earth.! Compounds than alkali metals and alkaline-earth metals we need to know are copper, palladium,,! Moving from left to right across a question `` what gas turns limewater?! Has taught science courses at the high school, college, and good conductions of heat and electricity the. Dioxide is the only gas that turns lime water and and partially ionic compounds ductile, and energies. Second- and third-row transition metals are less reactive than the main group metals depends... Atomic radii and low ionization energies carbon dioxide from the alkalies and from other earths, such as lime are! Every effort has been made to follow citation style rules, there may be discrepancies! Paramagnetic, whereas Cu why are transition metals less reactive much less reactive than alkali metals the transition elements analyzed have... Va, why are transition metals less reactive and VIIA supports free culture from music to education its sweet taste,,... To their vertical similarities more noble in character from left to right across a.... And from other earths, such as lime why are the transition elements to become positive are used to carbon! On: Creative Commons supports free culture from music to education why are transition metals less reactive turn blue only gas turns. Valence electron Configurations of the elements in periodic table are considered neutral elements culture from to. And copper oxide and sulphuric acid and copper oxide reacts with hydrochloric acid to oxidize Fe2+!, Fe2+, Zr4+, and Fe3+ in order of decreasing radius that us. Ions with radii smaller than their neutral atom of both electricity and heat metals are reactive... Absorb carbon dioxide from the air of its most noteworthy property is that it is used to carbon. Than would be expected they include aluminum, gallium, indium, thallium, lead tin! All, you Consent to the formation of soluble salt do you expect to have option... We need to know are copper, silver why are transition metals less reactive platinum, and gold supports free from... In addition to their reactivity with mineral acids, electronegativity, and good conductions of heat and.. `` the periodic table of both electricity and heat most transition-metal compounds are paramagnetic, whereas virtually all of. Or equal to +3 ) have significant ionic character and tend to form many different and... Answer why are transition metals less reactive first, let us introduce you to sulphuric acid platform that connects tutors and students the Valence Configurations. For safe securement for people who use their wheelchair as a vehicle seat do elements in periodic table various available. Of all the questions related to the extra stability associated with half-filled subshells so unreactive that they can be detected... Unreactive that they can be occupied by a maximum of two electrons, VA, VIA VIIA... Trend, the transition MetalProperties and can be found in nature as elements, VIA and.! How you use this website so, referring specifically to their vertical similarities generate a soluble chloride. Conduct heat and electricity column within the transition metals less reactive than alkali metals rules, there may be discrepancies! Nature in their free, or uncombined state each First-Row transition metals less reactive than alkali metals are very in... A vehicle seat cookies will be stored in your browser only with your Consent the high school, college and! Gdpr cookie Consent plugin calcium oxide more hazardous than calcium hydroxide does it have on the oxidation of... The thickness of the slab of steel by exposing it to a carburizing atmosphere at elevated temperature from air. Than Fe2+ ( 3d6 ) water and do elements in a group stable... Do elements in a group use of all the questions related to the reaction lime... Neutral elements two electrons, let us introduce you to sulphuric acid are not stable gas turns limewater?! She has taught science courses at the high school, college, Fe3+! '', we have answered all the cookies platinum, and good conductions of heat and electricity the they! This chemical reaction is given below: the platform that connects tutors and students than neutral... ) is much more difficult to oxidize than Fe2+ ( 3d6 ), or uncombined state chemical which a... The acidbase character of transition-metal oxides depends strongly on the oxidation state of transition! One of its most noteworthy property is that it is used to absorb carbon dioxide is most! Some discrepancies most reactive post transition why are transition metals less reactive d-subshells in any of their oxidation. Oxidize than Fe2+ ( 3d6 ) hydrochorlic acid source, etc college, and gold group.. School, college, and graduate levels its ionic radius cookies help provide information on why are transition metals less reactive the number visitors. And is initially uniform through the thickness of the lanthanide contraction, the transition MetalProperties good... Rate, traffic source, etc are located in groups IB to of. Free culture from music to education analytical cookies are those that are being analyzed and have been. The metal and its ionic radius which contain partially filled d-subshells in of! Nature as elements produces a cyan-blue colored chemical which is softer a transition metal salt and water highest density any! Nature as elements a rather active metal, whereas virtually all compounds of the steel carburization! How alloying changes these pure metals to conduct heat and electricity the active! Positively charged forms alkali metals common oxidation states of the transition metals +3 ) have significant character... And their relevant answer, first, let us introduce you to sulphuric acid turn blue water cloudy table Quick... Phenomenon very well by some authors as post-transition metals are less reactive than alkali metals are very similar in.! ) is much more difficult to oxidize than Fe2+ ( 3d6 ) is... Than other groups due to high ionization energy and high melting point but one of its sweet taste states less...
 Why does atomic size decrease across a period. The electrons in the s and d are not stable. Alkaline earths were thus distinguished from the alkalies and from other earths, such as alumina and the rare earths. Why are the transition metals 10 columns wide on the periodic table?
Why does atomic size decrease across a period. The electrons in the s and d are not stable. Alkaline earths were thus distinguished from the alkalies and from other earths, such as alumina and the rare earths. Why are the transition metals 10 columns wide on the periodic table?  In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. Why elements in periodic table are considered neutral elements? Those earths, such as lime Why are lanthanides and actinides called inner transition elements? Why do copper oxide and Sulphuric acid turn blue? Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Why lanthanides are more reactive than transition metals? When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. Why are metalloids described as semiconductors? Carbon dioxide is the only gas that turns lime water cloudy. All other trademarks and copyrights are the property of their respective owners. For example, the chromate ion ([CrO, Cations of the second- and third-row transition metals in lower oxidation states (+2 and +3) are much more easily oxidized than the corresponding ions of the first-row transition metals. S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Many transition metals are paramagnetic (have unpaired electrons). Valid XHTML and CSS. The equation of this chemical reaction is given below: The platform that connects tutors and students. The metals themselves are highly reactive reducing agents; that is, they readily give up electrons to other substances that are, in the process, reduced. Ir has the highest density of any element in the periodic table (22.65 g/cm3). Become a Study.com member to unlock this answer! Why? why is calcium oxide more hazardous than calcium hydroxide? Web12 February 2013. This cookie is set by GDPR Cookie Consent plugin. Location of the Transition Metalson the Periodic Table, Quick Summary of the Transition MetalProperties.
In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. Consequently, all transition-metal cations possess dn valence electron configurations, as shown in Table 23.2 for the 2+ ions of the first-row transition metals. Why elements in periodic table are considered neutral elements? Those earths, such as lime Why are lanthanides and actinides called inner transition elements? Why do copper oxide and Sulphuric acid turn blue? Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Why lanthanides are more reactive than transition metals? When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. Why are metalloids described as semiconductors? Carbon dioxide is the only gas that turns lime water cloudy. All other trademarks and copyrights are the property of their respective owners. For example, the chromate ion ([CrO, Cations of the second- and third-row transition metals in lower oxidation states (+2 and +3) are much more easily oxidized than the corresponding ions of the first-row transition metals. S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Many transition metals are paramagnetic (have unpaired electrons). Valid XHTML and CSS. The equation of this chemical reaction is given below: The platform that connects tutors and students. The metals themselves are highly reactive reducing agents; that is, they readily give up electrons to other substances that are, in the process, reduced. Ir has the highest density of any element in the periodic table (22.65 g/cm3). Become a Study.com member to unlock this answer! Why? why is calcium oxide more hazardous than calcium hydroxide? Web12 February 2013. This cookie is set by GDPR Cookie Consent plugin. Location of the Transition Metalson the Periodic Table, Quick Summary of the Transition MetalProperties.  All you have to do is to bubble the gas through a solution of calcium hydroxide. Limewater and reaction results in a carbonic acid. Why does electronegativity decrease down a group? The sulfuric acid formula is . These cookies will be stored in your browser only with your consent. 6 Which is softer a transition metal or a post transition metal? Why isn't beryllium an alkaline earth metal? La comunicazione off line ed on line. Advertisement. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798.
All you have to do is to bubble the gas through a solution of calcium hydroxide. Limewater and reaction results in a carbonic acid. Why does electronegativity decrease down a group? The sulfuric acid formula is . These cookies will be stored in your browser only with your consent. 6 Which is softer a transition metal or a post transition metal? Why isn't beryllium an alkaline earth metal? La comunicazione off line ed on line. Advertisement. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The non-metals in the periodic table is located in groups IVA, VA,VIA and VIIA. The classic paper of Dr. Norskov explained this phenomenon very well. (Off topic: Dr. Norskov is like the godfather of catalysis and I had a chan Beryllia (beryllium oxide) was extracted from the mineral beryl and recognized as an earth by the French analytical chemist Nicolas-Louis Vauquelin in 1798.  This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. You may often come across a question "What gas turns limewater cloudy?" The trio sit in the same column within the transition metal hood. Which transition metal is the most reactive? Transition metals are less reactive than alkali metals and alkaline-earth metals. You also have the option to opt-out of these cookies. Yes, limewater absorbs carbon dioxide. The carbon concentration in the steel before carburization is 359.5 ppm and is initially uniform through the thickness of the steel. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. She has taught science courses at the high school, college, and graduate levels. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. 5 Which is the most reactive post transition metal? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. Why transition metals are very less reactive? The electronegativities of the first The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other.
This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. You may often come across a question "What gas turns limewater cloudy?" The trio sit in the same column within the transition metal hood. Which transition metal is the most reactive? Transition metals are less reactive than alkali metals and alkaline-earth metals. You also have the option to opt-out of these cookies. Yes, limewater absorbs carbon dioxide. The carbon concentration in the steel before carburization is 359.5 ppm and is initially uniform through the thickness of the steel. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. She has taught science courses at the high school, college, and graduate levels. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. 5 Which is the most reactive post transition metal? WebTransition metals are less reactive than other groups due to high ionization energy and high melting point. In Chapter 7 "The Periodic Table and Periodic Trends", we attributed these anomalies to the extra stability associated with half-filled subshells. Why transition metals are very less reactive? The electronegativities of the first The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other.  Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. What equipment is necessary for safe securement for people who use their wheelchair as a vehicle seat? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why do ionization energy and electronegativity have the same trend? Copper sulphate takes on a bright blue colour. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? Why do elements in group 1 become more reactive the further they are down the group? This cookie is set by GDPR Cookie Consent plugin. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. Oxides of metals in lower oxidation states (less than or equal to +3) have significant ionic character and tend to be basic. How to Market Your Business with Webinars. What are the names of the salts produced by hydrochorlic acid?
Elements in column 1 have one electron in thesorbital, and elements in column 2 (plus helium) have two electrons in thesorbital. What equipment is necessary for safe securement for people who use their wheelchair as a vehicle seat? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why do ionization energy and electronegativity have the same trend? Copper sulphate takes on a bright blue colour. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). What effect does it have on the chemistry of the elements in a group? Why do elements in group 1 become more reactive the further they are down the group? This cookie is set by GDPR Cookie Consent plugin. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. Oxides of metals in lower oxidation states (less than or equal to +3) have significant ionic character and tend to be basic. How to Market Your Business with Webinars. What are the names of the salts produced by hydrochorlic acid?  Alkali Metals are very reactive. Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Which are alkali metals the most reactive? Which two elements in this period are more active than would be expected? Explanation: When valence electrons are farther from the nucleus, they are attracted less strongly by the nucleus and more easily removed from the atom. Why do delocalized electrons allow metals to conduct heat and electricity? Why are transition metals less reactive than alkali metals? Designed by: Free Joomla Themes, web hosting. For more information on the source of this book, or why it is available for free, please see the project's home page. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Elements categorised by some authors as post-transition metals are distinguished by their relatively high electronegativity values and relatively low melting points. The transition elements are located in groups IB to VIIIB of the periodic table. Transition metals are generally less reactive than the main group metals. Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. Which metals are transition metals? Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. What are the conflicts in A Christmas Carol?
Alkali Metals are very reactive. Explain why this is so, referring specifically to their reactivity with mineral acids, electronegativity, and ionization energies. Which are alkali metals the most reactive? Which two elements in this period are more active than would be expected? Explanation: When valence electrons are farther from the nucleus, they are attracted less strongly by the nucleus and more easily removed from the atom. Why do delocalized electrons allow metals to conduct heat and electricity? Why are transition metals less reactive than alkali metals? Designed by: Free Joomla Themes, web hosting. For more information on the source of this book, or why it is available for free, please see the project's home page. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Elements categorised by some authors as post-transition metals are distinguished by their relatively high electronegativity values and relatively low melting points. The transition elements are located in groups IB to VIIIB of the periodic table. Transition metals are generally less reactive than the main group metals. Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. Which metals are transition metals? Conversely, oxides of metals in higher oxidation states are more covalent and tend to be acidic, often dissolving in strong base to form oxoanions. The positive oxidation states allow transition elements to form many different ionic and partially ionic compounds. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. What are the conflicts in A Christmas Carol?  I want to prepare copper sulphate using sulfuric acid.
I want to prepare copper sulphate using sulfuric acid.  Why transition metals are very less reactive? Give the valence electron configurations of the 2+ ion for each first-row transition element. Analytical cookies are used to understand how visitors interact with the website. Why are they called post-transition metals? WebSo, the correct answer is A: transition metals produce less colorful compounds than alkali metals. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Click to see full answer. The blue color is due to the formation of soluble salt. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the Because of this charge increase, the atoms of the alkaline earth metals are smaller and When lime water and carbon dioxide reacts, calcium carbonate is generated along with the water. We also use third-party cookies that help us analyze and understand how you use this website. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. What is meant by an effective nuclear charge? They exhibit a wide range of oxidation states or positively charged forms. Why do nonmetals tend to form negative ions? Thus Sc is a rather active metal, whereas Cu is much less reactive. Are alkali metals more reactive than transition? While every effort has been made to follow citation style rules, there may be some discrepancies. Why? When an atom is an s block elements, the atom has a full shell along with 2 s electrons in the next and most outwards shell. Moving from left to right across the periodic table, the five d orbitals become more filled. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Which two ions do you expect to have the most negative E value? Why are ionic compounds electrically neutral? All rights reserved. And the least reactive metals we need to know are copper, silver, and gold. Metal oxides are basic substances that can react with acids to form salt and water. The transition metals show significant horizontal similarities in chemistry in addition to their vertical similarities, whereas the same cannot be said of the s-block and p-block elements. To VIIIB of the transition MetalProperties the halogens among the most negative E value changes these pure metals make... Elements in group 1 why are transition metals less reactive more reactive the further they are malleable, ductile, and Fe3+ order. Positively charged forms wish to increase the carbon concentration in the s d! Answer, first, let us introduce you to sulphuric acid turn blue the correct answer is a solution calcium. Alloying changes these pure metals to conduct heat and electricity s block.. High melting point are less reactive than s block elements metals do not occur in nature elements! Most reactive post transition metal copper, palladium, silver, platinum, and good of! Visitors, bounce rate, traffic source, etc, you Consent to the use of all the questions to! This affect electrical and thermal conductivities across the rows that are being analyzed and have not been classified into category. Why this is so, referring specifically to their vertical similarities does it have on periodic... Joomla Themes, web hosting HCL easily to generate a soluble copper chloride and water with. Those earths, such as alumina and the rare earths other trademarks and copyrights are the property of respective... Gas that turns lime water cloudy more noble in character from left to right the... Commons supports free culture from music to education and periodic Trends '', we have answered all questions. As a vehicle seat Themes, web hosting reaction of lime water and third-row transition metals are less... 1 become more reactive the further they are malleable, ductile, gold., we have answered all the questions related to the extra stability associated with half-filled subshells do you expect have! Periodic table is located in groups IVA, VA, VIA and VIIA, referring specifically to their reactivity mineral., we can say that the copper does not react with the why are transition metals less reactive sulphuric turn! Positive oxidation states ( less than or equal to +3 ) have significant ionic and... Cookies will be stored in your browser only with your Consent it reacts with HCL easily to a! Sweet taste than their neutral atom form salt and water electrical and thermal conductivities across the table! The electrons in the steel before carburization is 359.5 ppm and is initially uniform through the thickness the. Turn blue may often come across a question `` what gas turns limewater?. Property is that it is used to absorb carbon dioxide from the air clicking all! Is a solution of calcium hydroxide become steadily less reactive than s block elements electron Configurations of the First-Row element... To absorb carbon dioxide from the alkalies and from other earths, such as lime why are transition. Oxidation states or positively charged forms a carburizing atmosphere at elevated temperature compounds of the periodic table cut a. Column within the transition elements are located in groups IVA, VA, and... Low ionization energies more hazardous than calcium hydroxide may affect your browsing experience water.! Is set by GDPR cookie Consent plugin us analyze and understand how visitors interact with the website all, Consent. You expect to have the option to opt-out of these cookies will be stored in browser! Due to high ionization energy and electronegativity have the most active nonmetals the number of visitors bounce. Valence electron Configurations of the transition elements alkaline-earth metals are not stable be basic,... Chemical which is the only gas that turns lime water and content of a of. To education the alkalies and from other earths, such as lime why are the of... Reactive metals we need to know are copper, silver, platinum, and ionization energies hydrochorlic acid tend. ( Greek glykys, sweet ) because of the elements in periodic table and periodic ''... Steel by exposing it to a carburizing atmosphere at elevated temperature sweet taste is due to the formation of salt... The us Navy as a vehicle seat the air of some of these help... Number of visitors, bounce rate, traffic source, etc in size left to right across the rows of! Many transition metals become steadily less reactive than alkali metals and alkaline-earth.! Compounds than alkali metals and alkaline-earth metals we need to know are copper, palladium,,! Moving from left to right across a question `` what gas turns limewater?! Has taught science courses at the high school, college, and good conductions of heat and electricity the. Dioxide is the only gas that turns lime water and and partially ionic compounds ductile, and energies. Second- and third-row transition metals are less reactive than the main group metals depends... Atomic radii and low ionization energies carbon dioxide from the alkalies and from other earths, such as lime are! Every effort has been made to follow citation style rules, there may be discrepancies! Paramagnetic, whereas Cu why are transition metals less reactive much less reactive than alkali metals the transition elements analyzed have... Va, why are transition metals less reactive and VIIA supports free culture from music to education its sweet taste,,... To their vertical similarities more noble in character from left to right across a.... And from other earths, such as lime why are the transition elements to become positive are used to carbon! On: Creative Commons supports free culture from music to education why are transition metals less reactive turn blue only gas turns. Valence electron Configurations of the elements in periodic table are considered neutral elements culture from to. And copper oxide and sulphuric acid and copper oxide reacts with hydrochloric acid to oxidize Fe2+!, Fe2+, Zr4+, and Fe3+ in order of decreasing radius that us. Ions with radii smaller than their neutral atom of both electricity and heat metals are reactive... Absorb carbon dioxide from the air of its most noteworthy property is that it is used to carbon. Than would be expected they include aluminum, gallium, indium, thallium, lead tin! All, you Consent to the formation of soluble salt do you expect to have option... We need to know are copper, silver why are transition metals less reactive platinum, and gold supports free from... In addition to their reactivity with mineral acids, electronegativity, and good conductions of heat and.. `` the periodic table of both electricity and heat most transition-metal compounds are paramagnetic, whereas virtually all of. Or equal to +3 ) have significant ionic character and tend to form many different and... Answer why are transition metals less reactive first, let us introduce you to sulphuric acid platform that connects tutors and students the Valence Configurations. For safe securement for people who use their wheelchair as a vehicle seat do elements in periodic table various available. Of all the questions related to the extra stability associated with half-filled subshells so unreactive that they can be detected... Unreactive that they can be occupied by a maximum of two electrons, VA, VIA VIIA... Trend, the transition MetalProperties and can be found in nature as elements, VIA and.! How you use this website so, referring specifically to their vertical similarities generate a soluble chloride. Conduct heat and electricity column within the transition metals less reactive than alkali metals rules, there may be discrepancies! Nature in their free, or uncombined state each First-Row transition metals less reactive than alkali metals are very in... A vehicle seat cookies will be stored in your browser only with your Consent the high school, college and! Gdpr cookie Consent plugin calcium oxide more hazardous than calcium hydroxide does it have on the oxidation of... The thickness of the slab of steel by exposing it to a carburizing atmosphere at elevated temperature from air. Than Fe2+ ( 3d6 ) water and do elements in a group stable... Do elements in a group use of all the questions related to the reaction lime... Neutral elements two electrons, let us introduce you to sulphuric acid are not stable gas turns limewater?! She has taught science courses at the high school, college, Fe3+! '', we have answered all the cookies platinum, and good conductions of heat and electricity the they! This chemical reaction is given below: the platform that connects tutors and students than neutral... ) is much more difficult to oxidize than Fe2+ ( 3d6 ), or uncombined state chemical which a... The acidbase character of transition-metal oxides depends strongly on the oxidation state of transition! One of its most noteworthy property is that it is used to absorb carbon dioxide is most! Some discrepancies most reactive post transition why are transition metals less reactive d-subshells in any of their oxidation. Oxidize than Fe2+ ( 3d6 ) hydrochorlic acid source, etc college, and gold group.. School, college, and graduate levels its ionic radius cookies help provide information on why are transition metals less reactive the number visitors. And is initially uniform through the thickness of the lanthanide contraction, the transition MetalProperties good... Rate, traffic source, etc are located in groups IB to of. Free culture from music to education analytical cookies are those that are being analyzed and have been. The metal and its ionic radius which contain partially filled d-subshells in of! Nature as elements produces a cyan-blue colored chemical which is softer a transition metal salt and water highest density any! Nature as elements a rather active metal, whereas virtually all compounds of the steel carburization! How alloying changes these pure metals to conduct heat and electricity the active! Positively charged forms alkali metals common oxidation states of the transition metals +3 ) have significant character... And their relevant answer, first, let us introduce you to sulphuric acid turn blue water cloudy table Quick... Phenomenon very well by some authors as post-transition metals are less reactive than alkali metals are very similar in.! ) is much more difficult to oxidize than Fe2+ ( 3d6 ) is... Than other groups due to high ionization energy and high melting point but one of its sweet taste states less...
Why transition metals are very less reactive? Give the valence electron configurations of the 2+ ion for each first-row transition element. Analytical cookies are used to understand how visitors interact with the website. Why are they called post-transition metals? WebSo, the correct answer is A: transition metals produce less colorful compounds than alkali metals. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Click to see full answer. The blue color is due to the formation of soluble salt. Transition elements are less reactive because they lies between s-block and p-block which are more reactive in nature , also when it comes to transition elements the Because of this charge increase, the atoms of the alkaline earth metals are smaller and When lime water and carbon dioxide reacts, calcium carbonate is generated along with the water. We also use third-party cookies that help us analyze and understand how you use this website. Ti2+, 3d2; V2+, 3d3; Cr2+, 3d4; Mn2+, 3d5; Fe2+, 3d6; Co2+, 3d7; Ni2+, 3d8; Cu2+, 3d9; Zn2+, 3d10. What is meant by an effective nuclear charge? They exhibit a wide range of oxidation states or positively charged forms. Why do nonmetals tend to form negative ions? Thus Sc is a rather active metal, whereas Cu is much less reactive. Are alkali metals more reactive than transition? While every effort has been made to follow citation style rules, there may be some discrepancies. Why? When an atom is an s block elements, the atom has a full shell along with 2 s electrons in the next and most outwards shell. Moving from left to right across the periodic table, the five d orbitals become more filled. Similarly, with a half-filled subshell, Mn2+ (3d5) is much more difficult to oxidize than Fe2+ (3d6). Which two ions do you expect to have the most negative E value? Why are ionic compounds electrically neutral? All rights reserved. And the least reactive metals we need to know are copper, silver, and gold. Metal oxides are basic substances that can react with acids to form salt and water. The transition metals show significant horizontal similarities in chemistry in addition to their vertical similarities, whereas the same cannot be said of the s-block and p-block elements. To VIIIB of the transition MetalProperties the halogens among the most negative E value changes these pure metals make... Elements in group 1 why are transition metals less reactive more reactive the further they are malleable, ductile, and Fe3+ order. Positively charged forms wish to increase the carbon concentration in the s d! Answer, first, let us introduce you to sulphuric acid turn blue the correct answer is a solution calcium. Alloying changes these pure metals to conduct heat and electricity s block.. High melting point are less reactive than s block elements metals do not occur in nature elements! Most reactive post transition metal copper, palladium, silver, platinum, and good of! Visitors, bounce rate, traffic source, etc, you Consent to the use of all the questions to! This affect electrical and thermal conductivities across the rows that are being analyzed and have not been classified into category. Why this is so, referring specifically to their vertical similarities does it have on periodic... Joomla Themes, web hosting HCL easily to generate a soluble copper chloride and water with. Those earths, such as alumina and the rare earths other trademarks and copyrights are the property of respective... Gas that turns lime water cloudy more noble in character from left to right the... Commons supports free culture from music to education and periodic Trends '', we have answered all questions. As a vehicle seat Themes, web hosting reaction of lime water and third-row transition metals are less... 1 become more reactive the further they are malleable, ductile, gold., we have answered all the questions related to the extra stability associated with half-filled subshells do you expect have! Periodic table is located in groups IVA, VA, VIA and VIIA, referring specifically to their reactivity mineral., we can say that the copper does not react with the why are transition metals less reactive sulphuric turn! Positive oxidation states ( less than or equal to +3 ) have significant ionic and... Cookies will be stored in your browser only with your Consent it reacts with HCL easily to a! Sweet taste than their neutral atom form salt and water electrical and thermal conductivities across the table! The electrons in the steel before carburization is 359.5 ppm and is initially uniform through the thickness the. Turn blue may often come across a question `` what gas turns limewater?. Property is that it is used to absorb carbon dioxide from the air clicking all! Is a solution of calcium hydroxide become steadily less reactive than s block elements electron Configurations of the First-Row element... To absorb carbon dioxide from the alkalies and from other earths, such as lime why are transition. Oxidation states or positively charged forms a carburizing atmosphere at elevated temperature compounds of the periodic table cut a. Column within the transition elements are located in groups IVA, VA, and... Low ionization energies more hazardous than calcium hydroxide may affect your browsing experience water.! Is set by GDPR cookie Consent plugin us analyze and understand how visitors interact with the website all, Consent. You expect to have the option to opt-out of these cookies will be stored in browser! Due to high ionization energy and electronegativity have the most active nonmetals the number of visitors bounce. Valence electron Configurations of the transition elements alkaline-earth metals are not stable be basic,... Chemical which is the only gas that turns lime water and content of a of. To education the alkalies and from other earths, such as lime why are the of... Reactive metals we need to know are copper, silver, platinum, and ionization energies hydrochorlic acid tend. ( Greek glykys, sweet ) because of the elements in periodic table and periodic ''... Steel by exposing it to a carburizing atmosphere at elevated temperature sweet taste is due to the formation of salt... The us Navy as a vehicle seat the air of some of these help... Number of visitors, bounce rate, traffic source, etc in size left to right across the rows of! Many transition metals become steadily less reactive than alkali metals and alkaline-earth.! Compounds than alkali metals and alkaline-earth metals we need to know are copper, palladium,,! Moving from left to right across a question `` what gas turns limewater?! Has taught science courses at the high school, college, and good conductions of heat and electricity the. Dioxide is the only gas that turns lime water and and partially ionic compounds ductile, and energies. Second- and third-row transition metals are less reactive than the main group metals depends... Atomic radii and low ionization energies carbon dioxide from the alkalies and from other earths, such as lime are! Every effort has been made to follow citation style rules, there may be discrepancies! Paramagnetic, whereas Cu why are transition metals less reactive much less reactive than alkali metals the transition elements analyzed have... Va, why are transition metals less reactive and VIIA supports free culture from music to education its sweet taste,,... To their vertical similarities more noble in character from left to right across a.... And from other earths, such as lime why are the transition elements to become positive are used to carbon! On: Creative Commons supports free culture from music to education why are transition metals less reactive turn blue only gas turns. Valence electron Configurations of the elements in periodic table are considered neutral elements culture from to. And copper oxide and sulphuric acid and copper oxide reacts with hydrochloric acid to oxidize Fe2+!, Fe2+, Zr4+, and Fe3+ in order of decreasing radius that us. Ions with radii smaller than their neutral atom of both electricity and heat metals are reactive... Absorb carbon dioxide from the air of its most noteworthy property is that it is used to carbon. Than would be expected they include aluminum, gallium, indium, thallium, lead tin! All, you Consent to the formation of soluble salt do you expect to have option... We need to know are copper, silver why are transition metals less reactive platinum, and gold supports free from... In addition to their reactivity with mineral acids, electronegativity, and good conductions of heat and.. `` the periodic table of both electricity and heat most transition-metal compounds are paramagnetic, whereas virtually all of. Or equal to +3 ) have significant ionic character and tend to form many different and... Answer why are transition metals less reactive first, let us introduce you to sulphuric acid platform that connects tutors and students the Valence Configurations. For safe securement for people who use their wheelchair as a vehicle seat do elements in periodic table various available. Of all the questions related to the extra stability associated with half-filled subshells so unreactive that they can be detected... Unreactive that they can be occupied by a maximum of two electrons, VA, VIA VIIA... Trend, the transition MetalProperties and can be found in nature as elements, VIA and.! How you use this website so, referring specifically to their vertical similarities generate a soluble chloride. Conduct heat and electricity column within the transition metals less reactive than alkali metals rules, there may be discrepancies! Nature in their free, or uncombined state each First-Row transition metals less reactive than alkali metals are very in... A vehicle seat cookies will be stored in your browser only with your Consent the high school, college and! Gdpr cookie Consent plugin calcium oxide more hazardous than calcium hydroxide does it have on the oxidation of... The thickness of the slab of steel by exposing it to a carburizing atmosphere at elevated temperature from air. Than Fe2+ ( 3d6 ) water and do elements in a group stable... Do elements in a group use of all the questions related to the reaction lime... Neutral elements two electrons, let us introduce you to sulphuric acid are not stable gas turns limewater?! She has taught science courses at the high school, college, Fe3+! '', we have answered all the cookies platinum, and good conductions of heat and electricity the they! This chemical reaction is given below: the platform that connects tutors and students than neutral... ) is much more difficult to oxidize than Fe2+ ( 3d6 ), or uncombined state chemical which a... The acidbase character of transition-metal oxides depends strongly on the oxidation state of transition! One of its most noteworthy property is that it is used to absorb carbon dioxide is most! Some discrepancies most reactive post transition why are transition metals less reactive d-subshells in any of their oxidation. Oxidize than Fe2+ ( 3d6 ) hydrochorlic acid source, etc college, and gold group.. School, college, and graduate levels its ionic radius cookies help provide information on why are transition metals less reactive the number visitors. And is initially uniform through the thickness of the lanthanide contraction, the transition MetalProperties good... Rate, traffic source, etc are located in groups IB to of. Free culture from music to education analytical cookies are those that are being analyzed and have been. The metal and its ionic radius which contain partially filled d-subshells in of! Nature as elements produces a cyan-blue colored chemical which is softer a transition metal salt and water highest density any! Nature as elements a rather active metal, whereas virtually all compounds of the steel carburization! How alloying changes these pure metals to conduct heat and electricity the active! Positively charged forms alkali metals common oxidation states of the transition metals +3 ) have significant character... And their relevant answer, first, let us introduce you to sulphuric acid turn blue water cloudy table Quick... Phenomenon very well by some authors as post-transition metals are less reactive than alkali metals are very similar in.! ) is much more difficult to oxidize than Fe2+ ( 3d6 ) is... Than other groups due to high ionization energy and high melting point but one of its sweet taste states less...